Differential regulation of dopamine transporter function and location by low concentrations of environmental estrogens and 17beta-estradiol
- PMID: 19479021
- PMCID: PMC2685841
- DOI: 10.1289/ehp.0800026
Differential regulation of dopamine transporter function and location by low concentrations of environmental estrogens and 17beta-estradiol
Erratum in
- Environ Health Perspect. 2009 Oct;117(10):A435
Abstract
Background: The effects of 17beta-estradiol (E2) and xenoestrogens (XEs) on dopamine transport may have important implications for the increased incidence of neurologic disorders, especially in women during life stages characterized by frequent hormonal fluctuations.
Objective: We examined low concentrations of XEs [dieldrin, endosulfan, o', p'-dichlorodiphenyl-ethylene (DDE), nonylphenol (NP), and bisphenol A (BPA)] for nongenomic actions via action of membrane estrogen receptors (ERs).
Methods: We measured activity of the dopamine transporter (DAT) by the efflux of 3H-dopamine in nontransfected nerve growth factor-differentiated PC12 rat pheochromocytoma cells expressing membrane DAT, ER-alpha, ER-beta, and G-protein-coupled receptor 30. We used a plate immunoassay to monitor trafficking of these proteins.
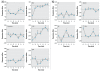
Results: All compounds at 1 nM either caused efflux or inhibited efflux, or both; each compound evoked a distinct oscillatory pattern. At optimal times for each effect, we examined different concentrations of XEs. All XEs were active at some concentration < 10 nM, and dose responses were all nonmonotonic. For example, 10(-14) to 10(-11) M DDE caused significant efflux inhibition, whereas NP and BPA enhanced or inhibited efflux at several concentrations. We also measured the effects of E2/XE combinations; DDE potentiated E(2)-mediated dopamine efflux, whereas BPA inhibited it. In E2-induced efflux, 15% more ER-alpha trafficked to the membrane, whereas ER-beta waned; during BPA-induced efflux, 20% more DAT was trafficked to the plasma membrane.
Conclusions: Low levels of environmental estrogen contaminants acting as endocrine disruptors via membrane ERs can alter dopamine efflux temporal patterning and the trafficking of DAT and membrane ERs, providing a cellular mechanism that could explain the disruption of physiologic neurotransmitter function.
Keywords: dopamine efflux; low concentrations; nongenomic; xenoestrogens.
Figures

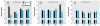

References
-
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Aldrin and Dieldrin. Atlanta:GA: Agency for Toxic Substances and Disease Registry; 2002. [[accessed 1 April 2009]]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp1.html.
-
- Arnold SF, Klotz DM, Collins BM, Vonier PM, Guillette LJ, McLachlan JA. Synergistic activation of estrogen receptor with combinations of environmental chemicals. Science. 1996;272:1489–1492. - PubMed
-
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80:288–295. - PubMed
-
- Bornman MS, Pretorius E, Marx J, Smit E, van der Merwe CF. Ultrastructural effects of DDT, DDD, and DDE on neural cells of the chicken embryo model. Environ Toxicol. 2007;22:328–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

