Zidovudine/lamivudine for HIV-1 infection contributes to limb fat loss
- PMID: 19479079
- PMCID: PMC2682584
- DOI: 10.1371/journal.pone.0005647
Zidovudine/lamivudine for HIV-1 infection contributes to limb fat loss
Abstract
Background: Lipoatrophy is known to be associated with stavudine as part of the treatment for HIV infection, but it is less clear if this serious side effect is also related to other nucleoside reverse transcriptase inhibitors like zidovudine. We aimed to determine whether zidovudine-sparing first-line antiretroviral therapy would lead to less lipoatrophy and other metabolic changes than zidovudine-containing therapy.
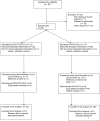
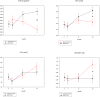
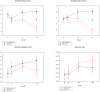
Methodology/principal findings: Fifty antiretroviral therapy-naïve HIV-1 infected men with an indication to start antiretroviral therapy were included in a randomized single blinded clinical trial. Randomisation was between zidovudine-containing therapy (zidovudine/lamivudine+lopinavir/ritonavir) and zidovudine-sparing therapy (nevirapine+lopinavir/ritonavir). Main outcome measures were body composition assessed by computed tomography and dual-energy X-ray absorptiometry scan and lipid profile before and after 3, 12, 24 months of antiretroviral therapy. In the zidovudine/lamivudine+lopinavir/ritonavir group, from 3 months onward limb fat decreased progressively by 684+/-293 grams (estimated mean+/-standard error of the mean)(p = 0.02) up to 24 months whereas abdominal fat increased, but exclusively in the visceral compartment (+21.9+/-8.1 cm(2), p = 0.008)). In contrast, in the nevirapine+lopinavir/ritonavir group, a generalized increase in fat mass was observed. After 24 months no significant differences in high density lipoprotein and total/high density lipoprotein cholesterol ratio were found between both treatment groups, but total and low density lipoprotein cholesterol levels were higher in the nevirapine+lopinavir/ritonavir group (6.1+/-0.2 versus 5.3+/-0.2 and 3.6+/-0.1 versus 2.8+/-0.1 mmol/l respectively, p<0.05). Virologic response and safety were comparable in both groups.
Conclusions/significance: Zidovudine/lamivudine+lopinavir/ritonavir, but not nevirapine+lopinavir/ritonavir in antiretroviral therapy-naïve patients, is associated with lipoatrophy and greater relative intraabdominal lipohypertrophy, suggesting that zidovudine/lamivudine contributes to both these features of lipodystrophy. These findings support to no longer consider zidovudine/lamivudine as one of the preferred possible components of first-line antiretroviral therapy where alternative treatments are available.
Trial registration: ClinicalTrials.gov NCT 00122226.
Trial registration: ClinicalTrials.gov NCT00122226.
Conflict of interest statement
Figures
References
-
- Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. - PubMed
-
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. - PubMed
-
- Podzamczer D, Ferrer E, Sanchez P, Gatell JM, Crespo M, et al. Less lipoatrophy and better lipid profile with abacavir as compared to stavudine: 96-week results of a randomized study. J Acquir Immune Defic Syndr. 2007;44:139–147. - PubMed
-
- Dube MP, Komarow L, Mulligan K, Grinspoon SK, Parker RA, et al. Long-term body fat outcomes in antiretroviral-naive participants randomized to nelfinavir or efavirenz or both plus dual nucleosides. Dual X-ray absorptiometry results from A5005s, a substudy of Adult Clinical Trials Group 384. J Acquir Immune Defic Syndr. 2007;45:508–514. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

