Complete and sustained remission of juvenile dermatomyositis resulting from aggressive treatment
- PMID: 19479872
- PMCID: PMC4254704
- DOI: 10.1002/art.24571
Complete and sustained remission of juvenile dermatomyositis resulting from aggressive treatment
Abstract
Objective: To assess the time needed to achieve sustained, medication-free remission in a cohort of patients with juvenile dermatomyositis (DM) receiving a stepwise, aggressive treatment protocol.
Methods: Between 1994 and 2004, a cohort of 49 children with juvenile DM who were followed up at a single tertiary care children's hospital using disease activity measures according to a specific protocol received standardized therapy with steroids and methotrexate. If a patient's strength or muscle enzyme levels did not normalize with this initial therapy, additional medications were added in rapid succession to the treatment regimen. The primary outcome measure was time to complete remission. Additional outcome measures were onset of calcinosis, effect of treatment on height, and complications resulting from medications.
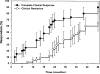
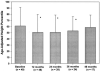
Results: Forty-nine patients were followed up for a mean+/-SD of 48+/-30 months. All but 1 patient received 2 or more medications simultaneously. Transient localized calcifications occurred in 4 patients (8%), and 2 additional patients (4%) had persistent calcinosis. Despite the aggressive therapy, complications associated with treatment were mild and were primarily attributable to steroids. No persistent effect on longitudinal growth was observed. A complete, medication-free remission was achieved in 28 patients; the median time to achievement of complete remission was 38 months (95% confidence interval 32-44 months). None of these patients experienced a disease flare that required resumption of medications during the subsequent period of observation (mean+/-SD 36+/-19.7 months).
Conclusion: Our findings suggest that aggressive treatment of juvenile DM aimed at achieving rapid, complete control of muscle weakness and inflammation improves outcomes and reduces disease-related complications. In more than one-half of the children whose disease was treated in this manner (28 of 49), a prolonged, medication-free remission was attained within a median of 38 months from the time of diagnosis.
Figures
References
-
- Bitnum S, Daeschner CW, Jr, Travis LB, Dodge WF, Hopps HC. Dermatomyositis. J Pediatr. 1964;64:101–31. - PubMed
-
- Ramanan AV, Campbell-Webster N, Ota S, Parker S, Tran D, Tyrrell PN, et al. The effectiveness of treating juvenile dermatomyositis with methotrexate and aggressively tapered corticosteroids. Arthritis Rheum. 2005;52:3570–8. - PubMed
-
- Al-Mayouf SM, Laxer RM, Schneider R, Silverman ED, Feldman BM. Intravenous immunoglobulin therapy for juvenile dermatomyositis: efficacy and safety. J Rheumatol. 2000;27:2498–503. - PubMed
-
- Huber AM, Lang B, LeBlanc CM, Birdi N, Bolaria RK, Malleson P, et al. Medium- and long-term functional outcomes in a multi-center cohort of children with juvenile dermatomyositis. Arthritis Rheum. 2000;43:541–9. - PubMed
-
- Bowyer SL, Blane CE, Sullivan DB, Cassidy JT. Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr. 1983;103:882–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

