Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy
- PMID: 19479879
- PMCID: PMC2697261
- DOI: 10.1002/art.24555
Elevated serum interferon-alpha activity in juvenile dermatomyositis: associations with disease activity at diagnosis and after thirty-six months of therapy
Abstract
Objective: Interferon-alpha (IFNalpha) has been implicated in the pathogenesis of juvenile dermatomyositis (DM). The aim of this study was to examine serum IFNalpha activity in a cohort of children with juvenile DM to determine relationships between IFNalpha and indicators of disease activity and severity.
Methods: Thirty-nine children with definite/probable juvenile DM were included in the study. Serum samples were obtained at the time of diagnosis from 18 untreated patients with juvenile DM. Second samples from 11 of these patients were obtained at 24 months, while they were receiving treatment, and third samples were obtained from 7 of these patients at 36 months. The remaining 21 children were studied 36 months after their initial diagnosis. Serum IFNalpha activity was measured using a functional reporter cell assay.
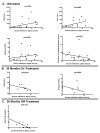
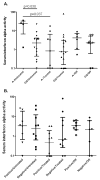
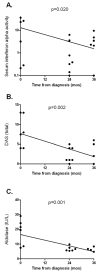
Results: Patients with juvenile DM had higher serum IFNalpha activity than both pediatric and adult healthy control subjects. In untreated patients, serum IFNalpha activity was positively correlated with serum muscle enzyme levels (P<0.05 for creatine kinase, aspartate aminotransferase, and aldolase) and inversely correlated with the duration of untreated disease (P=0.017). The tumor necrosis factor alpha -308A allele was associated with higher serum IFNalpha levels only in untreated patients (P=0.030). At 36 months, serum IFNalpha levels were inversely correlated with muscle enzyme levels in those patients still requiring therapy and with the skin Disease Activity Score in those patients who had completed therapy (P=0.002).
Conclusion: Serum IFNalpha activity was associated with higher serum levels of muscle-derived enzymes and a shorter duration of untreated disease in patients with newly diagnosed juvenile DM and was inversely correlated with measures of chronic disease activity at 36 months postdiagnosis. These data suggest that IFNalpha could play a role in disease initiation in juvenile DM.
Figures


Similar articles
-
TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications.Arthritis Rheum. 2000 Oct;43(10):2368-77. doi: 10.1002/1529-0131(200010)43:10<2368::AID-ANR26>3.0.CO;2-8. Arthritis Rheum. 2000. PMID: 11037898
-
Lesional and nonlesional skin from patients with untreated juvenile dermatomyositis displays increased numbers of mast cells and mature plasmacytoid dendritic cells.Arthritis Rheum. 2010 Sep;62(9):2813-22. doi: 10.1002/art.27529. Arthritis Rheum. 2010. PMID: 20506305 Free PMC article.
-
Association of normal nailfold end row loop numbers with a shorter duration of untreated disease in children with juvenile dermatomyositis.Arthritis Rheum. 2010 May;62(5):1533-8. doi: 10.1002/art.27379. Arthritis Rheum. 2010. PMID: 20213809 Free PMC article.
-
Cytokines in juvenile dermatomyositis pathophysiology: potential and challenge.Curr Opin Rheumatol. 2003 Nov;15(6):691-7. doi: 10.1097/00002281-200311000-00003. Curr Opin Rheumatol. 2003. PMID: 14569197 Review.
-
2016 American College of Rheumatology/European League Against Rheumatism Criteria for Minimal, Moderate, and Major Clinical Response in Juvenile Dermatomyositis: An International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology International Trials Organisation Collaborative Initiative.Arthritis Rheumatol. 2017 May;69(5):911-923. doi: 10.1002/art.40060. Epub 2017 Apr 6. Arthritis Rheumatol. 2017. PMID: 28382778 Free PMC article.
Cited by
-
Linkage of type I interferon activity and TNF-alpha levels in serum with sarcoidosis manifestations and ancestry.PLoS One. 2011;6(12):e29126. doi: 10.1371/journal.pone.0029126. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22195005 Free PMC article.
-
Immunological biomarkers in dermatomyositis.Curr Rheumatol Rep. 2015 Nov;17(11):68. doi: 10.1007/s11926-015-0543-y. Curr Rheumatol Rep. 2015. PMID: 26404949 Review.
-
The type I interferons: Basic concepts and clinical relevance in immune-mediated inflammatory diseases.Gene. 2016 Jan 15;576(1 Pt 1):14-21. doi: 10.1016/j.gene.2015.09.058. Epub 2015 Sep 26. Gene. 2016. PMID: 26410416 Free PMC article. Review.
-
Decreased Type I Interferon Production by Plasmacytoid Dendritic Cells Contributes to Severe Dengue.Front Immunol. 2020 Dec 17;11:605087. doi: 10.3389/fimmu.2020.605087. eCollection 2020. Front Immunol. 2020. PMID: 33391269 Free PMC article.
-
JAK inhibitors: a potential treatment for JDM in the context of the role of interferon-driven pathology.Pediatr Rheumatol Online J. 2021 Sep 25;19(1):146. doi: 10.1186/s12969-021-00637-8. Pediatr Rheumatol Online J. 2021. PMID: 34563217 Free PMC article. Review.
References
-
- Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371(9631):2201–12. - PubMed
-
- Reed AM, Pachman L, Ober C. Molecular genetic studies of major histocompatibility complex genes in children with juvenile dermatomyositis: increased risk associated with HLA-DQA1 *0501. Hum Immunol. 1991;32(4):235–40. - PubMed
-
- Pachman LM, Liotta-Davis MR, Hong DK, Kinsella TR, Mendez EP, Kinder JM, et al. TNFalpha-308A allele in juvenile dermatomyositis: association with increased production of tumor necrosis factor alpha, disease duration, and pathologic calcifications. Arthritis Rheum. 2000;43(10):2368–77. - PubMed
-
- Manlhiot C, Liang L, Tran D, Bitnun A, Tyrrell PN, Feldman BM. Assessment of an infectious disease history preceding juvenile dermatomyositis symptom onset. Rheumatology (Oxford) 2008;47(4):526–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

