Oxidative stress disrupts oligodendrocyte maturation
- PMID: 19479983
- PMCID: PMC3138415
- DOI: 10.1002/jnr.22139
Oxidative stress disrupts oligodendrocyte maturation
Abstract
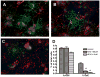
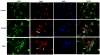
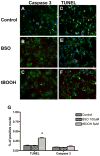
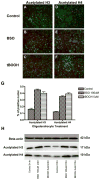
Periventricular white matter injury (PWMI) is the leading cause of chronic neurologic injury among survivors of preterm birth. The hallmark of PWMI is hypomyelination and a lack of mature, myelinating oligodendrocytes. Oligodendrocytes undergo a well-characterized lineage progression from neural stem cell to mature oligodendrocyte. Oligodendrocyte precursors have increased susceptibility to oxidative and free radical-mediated injury compared with mature oligodendrocytes as a result of lower levels of antioxidant enzymes and free radical scavengers. In this study, we show that oxidative stress disrupts oligodendrocyte differentiation by two mechanisms. First, oxidizing agents decrease the expression of key genes that promote oligodendrocyte differentiation from neural stem cells and increase the expression of genes known to inhibit differentiation. Second, global histone acetylation persists under conditions of oxidative stress, further contributing to the prevention of oligodendrocyte differentiation. Both of these mechanisms result in the arrest of oligodendrocyte differentiation without an increase in cell death.
(c) 2009 Wiley-Liss, Inc.
Figures
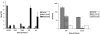
References
-
- Altman J, Bayer SA. The development of the rat spinal cord. In: Beck F, Hild W, van Limborgh J, Ortmann R, Pauly JE, Schiebler TH, editors. Advances in anatomy, embryology and cell biology. Vol. 85. Berlin: Springer Verlag; 1984. - PubMed
-
- Back SA, Volpe JJ, Kinney HH. Immunocytochemical characterization of oligodendrocyte development in human cerebral white matter. Soc Neurosci Abstr. 1996;20:1722.
-
- Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

