Establishment of trophectoderm and inner cell mass lineages in the mouse embryo
- PMID: 19479991
- PMCID: PMC2874917
- DOI: 10.1002/mrd.21057
Establishment of trophectoderm and inner cell mass lineages in the mouse embryo
Abstract
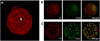
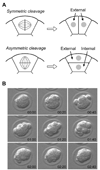
The first cell lineage specification in mouse embryo development is the formation of trophectoderm (TE) and inner cell mass (ICM) of the blastocyst. This article is to review and discuss the current knowledge on the cellular and molecular mechanisms of this particular event. Several transcription factors have been identified as the critical regulators of the formation or maintenance of the two cell lineages. The establishment of TE manifests as the formation of epithelium, and is dependent on many structural and regulatory components that are commonly found and that function in many epithelial tissues. Distinct epithelial features start to emerge at the late 8-cell stage, but the fates of blastomeres are not fixed as TE or ICM until around 32-cell stage. The location of blastomeres at this stage, that is, external or internal of the embryo, in effect defines the commitment towards the TE or ICM lineage, respectively. Some studies implicate the presence of a developmental bias among blastomeres at 2- or 4-cell stage, although it is unlikely to play a decisive role in the establishment of TE and ICM. The unique mode of cell lineage specification in the mouse embryo is further discussed in comparison with the formation of initial cell lineages, namely the three germ layers, in non-mammalian embryos.
Figures


References
-
- Alarcón VB, Marikawa Y. Deviation of the blastocyst axis from the first cleavage plane does not affect the quality of mouse postimplantation development. Biol Reprod. 2003;69:1208–1212. - PubMed
-
- Alarcón VB, Marikawa Y. Unbiased contribution of the first two blastomeres to mouse blastocyst development. Mol Reprod Dev. 2005;72:354–361. - PubMed
-
- Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. - PubMed
-
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

