Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial
- PMID: 19480232
- PMCID: PMC2675900
- DOI: 10.1093/sleep/32.5.648
Comparison of mandibular advancement splint and tongue stabilizing device in obstructive sleep apnea: a randomized controlled trial
Erratum in
- Sleep. 2009 Aug 1;32(8):table of contents
Abstract
Study objectives: To compare the efficacy of a mandibular advancement splint (MAS) and a novel tongue stabilizing device (TSD) in the treatment of obstructive sleep apnea (OSA).
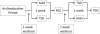
Design: A randomized crossover design was used.
Patients: Twenty-seven patients (20 male, 7 female), recruited from a tertiary hospital sleep clinic.
Measurements and results: The apnea-hypopnea index (AHI) was reduced with MAS (11.68 +/- 8.94, P = 0.000) and TSD (13.15 +/- 10.77, P = 0.002) compared with baseline (26.96 +/- 17.17). The arousal index decreased for MAS (21.09 +/- 9.27, P = 0.004) and TSD (21.9 +/- 10.56, P = 0.001) compared with baseline (33.23 +/- 16.41). Sixty-eight percent of patients achieved a complete or partial response with MAS, compared with 45% with TSD. The Epworth Sleepiness Scale (ESS) score was decreased with MAS (P = < 0.001) and TSD (P = 0.002). Subjective improvements in snoring and quality of sleep were reported, with a better response for MAS than TSD. Compliance was poorer for TSD, and the side effect profiles of the 2 modalities were different. All patients were satisfied with MAS compared to TSD, and 91% of patients preferred the MAS.
Conclusion: Objective testing showed the MAS and TSD had similar efficacy in terms of AHI reduction. Patients reported improvements with both devices; however, better compliance and a clear preference for MAS was apparent when both devices were offered. Longer term studies are needed to clarify the role of TSD.
Figures



References
-
- Cistulli P, Sullivan C, editors. In: Sleep and breathing. New York: Marcel Dekker Inc; 1994. Pathophysiology of sleep apnea.
-
- Anonymous. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement in clinical research. Sleep. 1999;22:667–89. - PubMed
-
- Qureshi A, Ballard RD. Obstructive sleep apnea. J Allergy Immunol. 2003;112:643–51. - PubMed
-
- Redline S. Epidemiology of sleep-disordered breathing. Semin Respir Crit Care Med. 1998;19:113–22.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

