Proteomic analysis of DNA-protein cross-linking by antitumor nitrogen mustards
- PMID: 19480393
- PMCID: PMC2706101
- DOI: 10.1021/tx900078y
Proteomic analysis of DNA-protein cross-linking by antitumor nitrogen mustards
Erratum in
- Chem Res Toxicol. 2010 Aug 16;23(8):1427
Abstract
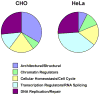
Nitrogen mustards are antitumor agents used clinically for the treatment of a variety of neoplastic conditions. The biological activity of these compounds is typically attributed to their ability to induce DNA-DNA cross-links. However, nitrogen mustards are able to produce a variety of other lesions, including DNA-protein cross-links (DPCs). DPCs induced by nitrogen mustards are not well-characterized because of their structural complexity and the insufficient specificity and sensitivity of previously available experimental methodologies. In the present work, affinity capture methodology in combination with mass spectrometry-based proteomics was employed to identify mammalian proteins that form covalent cross-links to DNA in the presence of a simple nitrogen mustard, mechlorethamine. Following incubation of 5'-biotinylated DNA duplexes with nuclear protein extracts, DPCs were isolated by affinity capture on streptavidin beads, and the cross-linked proteins were identified by high-performance liquid chromatography-electrospray tandem mass spectrometry of tryptic peptides. Mechlorethamine treatment resulted in the formation of DPCs with nuclear proteins involved in chromatin regulation, DNA replication and repair, cell cycle control, transcriptional regulation, and cell architecture. Western blot analysis was employed to confirm protein identification and to quantify the extent of drug-mediated cross-linking. Mass spectrometry of amino acid-nucleobase conjugates found in total proteolytic digests revealed that mechlorethamine-induced DPCs are formed via alkylation of the N7 position of guanine in duplex DNA and cysteine thiols within the proteins to give N-[2-[S-cysteinyl]ethyl]-N-[2-(guan-7-yl)ethyl]methylamine lesions. The results described herein suggest that cellular exposure to nitrogen mustards leads to cross-linking of a large spectrum of nuclear proteins to chromosomal DNA, potentially contributing to the cytotoxic and mutagenic effects of these drugs.
Figures






Similar articles
-
Cross-linking of the DNA repair protein Omicron6-alkylguanine DNA alkyltransferase to DNA in the presence of antitumor nitrogen mustards.Chem Res Toxicol. 2008 Apr;21(4):787-95. doi: 10.1021/tx7004508. Epub 2008 Feb 14. Chem Res Toxicol. 2008. PMID: 18324787 Free PMC article.
-
Covalent DNA-Protein Cross-Linking by Phosphoramide Mustard and Nornitrogen Mustard in Human Cells.Chem Res Toxicol. 2016 Feb 15;29(2):190-202. doi: 10.1021/acs.chemrestox.5b00430. Epub 2016 Jan 20. Chem Res Toxicol. 2016. PMID: 26692166 Free PMC article.
-
Mechlorethamine-induced DNA-protein cross-linking in human fibrosarcoma (HT1080) cells.J Proteome Res. 2011 Jun 3;10(6):2785-96. doi: 10.1021/pr200042u. Epub 2011 Apr 29. J Proteome Res. 2011. PMID: 21486066 Free PMC article.
-
Therapeutic journery of nitrogen mustard as alkylating anticancer agents: Historic to future perspectives.Eur J Med Chem. 2018 May 10;151:401-433. doi: 10.1016/j.ejmech.2018.04.001. Epub 2018 Apr 3. Eur J Med Chem. 2018. PMID: 29649739 Review.
-
Mass Spectrometry-Based Tools to Characterize DNA-Protein Cross-Linking by Bis-Electrophiles.Basic Clin Pharmacol Toxicol. 2017 Sep;121 Suppl 3(Suppl 3):63-77. doi: 10.1111/bcpt.12751. Epub 2017 Mar 14. Basic Clin Pharmacol Toxicol. 2017. PMID: 28032943 Free PMC article. Review.
Cited by
-
Jak-STAT Inhibition Mediates Romidepsin and Mechlorethamine Synergism in Cutaneous T-Cell Lymphoma.J Invest Dermatol. 2021 Dec;141(12):2908-2920.e7. doi: 10.1016/j.jid.2021.04.023. Epub 2021 Jun 3. J Invest Dermatol. 2021. PMID: 34089720 Free PMC article.
-
Bypass of DNA-Protein Cross-links Conjugated to the 7-Deazaguanine Position of DNA by Translesion Synthesis Polymerases.J Biol Chem. 2016 Nov 4;291(45):23589-23603. doi: 10.1074/jbc.M116.745257. Epub 2016 Sep 12. J Biol Chem. 2016. PMID: 27621316 Free PMC article.
-
Mass spectrometric methods for the analysis of nucleoside-protein cross-links: application to oxopropenyl-deoxyadenosine.Chem Res Toxicol. 2014 Jan 21;27(1):136-46. doi: 10.1021/tx400384e. Epub 2013 Dec 20. Chem Res Toxicol. 2014. PMID: 24359270 Free PMC article.
-
A quantitative PCR-based assay reveals that nucleotide excision repair plays a predominant role in the removal of DNA-protein crosslinks from plasmids transfected into mammalian cells.DNA Repair (Amst). 2018 Feb;62:18-27. doi: 10.1016/j.dnarep.2018.01.004. Epub 2018 Jan 9. DNA Repair (Amst). 2018. PMID: 29413806 Free PMC article.
-
Enzymatic bypass of an N6-deoxyadenosine DNA-ethylene dibromide-peptide cross-link by translesion DNA polymerases.J Biol Chem. 2021 Jan-Jun;296:100444. doi: 10.1016/j.jbc.2021.100444. Epub 2021 Feb 20. J Biol Chem. 2021. PMID: 33617883 Free PMC article.
References
-
- Barker S, Weinfeld M, Zheng J, Li L, Murray D. Identification of mammalian proteins cross-linked to DNA by ionizing radiation. J Biol Chem. 2005;280:33826–33838. - PubMed
-
- Ewig RA, Kohn KW. DNA damage and repair in mouse leukemia L1210 cells treated with nitrogen mustard, 1,3-bis(2-chloroethyl)-1-nitrosourea, and other nitrosoureas. Cancer Res. 1977;37:2114–2122. - PubMed
-
- Kloster M, Kostrhunova H, Zaludova R, Malina J, Kasparkova J, Brabec V, Farrell N. Trifunctional dinuclear platinum complexes as DNA-protein cross-linking agent. Biochemistry. 2004;43:7776–7786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

