Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs
- PMID: 19480567
- PMCID: PMC3135180
- DOI: 10.1089/scd.2009.0113
Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs
Abstract
Coordinated transcription factor networks have emerged as the master regulatory mechanisms of stem cell pluripotency and differentiation. Many stem cell-specific transcription factors, including the pluripotency transcription factors, OCT4, NANOG, and SOX2 function in combinatorial complexes to regulate the expression of loci, which are involved in embryonic stem (ES) cell pluripotency and cellular differentiation. This review will address how these pathways form a reciprocal regulatory circuit whereby the equilibrium between stem cell self-renewal, proliferation, and differentiation is in perpetual balance. We will discuss how distinct epigenetic repressive pathways involving polycomb complexes, DNA methylation, and microRNAs cooperate to reduce transcriptional noise and to prevent stochastic and aberrant induction of differentiation. We will provide a brief overview of how these networks cooperate to modulate differentiation along hematopoietic and neuronal lineages. Finally, we will describe how aberrant functioning of components of the stem cell regulatory network may contribute to malignant transformation of adult stem cells and the establishment of a "cancer stem cell" phenotype and thereby underlie multiple types of human malignancies.
Figures
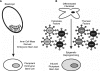
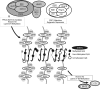
 ), or fully methylated (•).
), or fully methylated (•).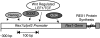
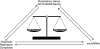
References
-
- Chen AC. Gudas LJ. An analysis of retinoic acid-induced gene expression and metabolism in AB1 embryonic stem cells. J Biol Chem. 1996;271:14971–14980. - PubMed
-
- Mongan NP. Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. - PubMed
-
- Evans MJ. Kaufman MH. Establishment in culture of pluripotent cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

