Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress
- PMID: 19480571
- PMCID: PMC2792062
- DOI: 10.1089/ten.TEA.2008.0444
Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress
Abstract
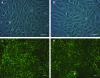
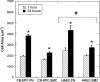
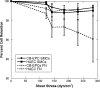
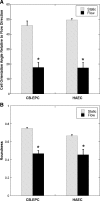
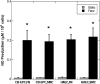
Endothelial progenitor cells isolated from umbilical cord blood (CB-EPCs) represent a promising source of endothelial cells for synthetic vascular grafts and tissue-engineered blood vessels since they are readily attainable, can be easily isolated, and possess a high proliferation potential. The objective of this study was to compare the functional behavior of late outgrowth CB-EPCs with human aortic endothelial cells (HAECs). CB-EPCs and HAECs were cultured on either smooth muscle cells in a coculture model of a tissue-engineered blood vessels or fibronectin adsorbed to Teflon-AF-coated glass slides. Late outgrowth CB-EPCs expressed endothelial cell-specific markers and were negative for the monocytic marker CD14. CB-EPCs have higher proliferation rates than HAECs, but are slightly smaller in size. CB-EPCs remained adherent under supraphysiological shear stresses, oriented and elongated in the direction of flow, and expressed similar numbers of alpha(5)beta(1) and alpha(v)beta(3) integrins and antithrombotic genes compared to HAECs. There were some differences in mRNA levels of E-selectin and vascular cell adhesion molecule 1 between CB-EPCs and HAECs; however, protein levels were similar on the two cell types, and CB-EPCs did not support adhesion of monocytes in the absence of tumor necrosis factor-alpha stimulation. Although CB-EPCs expressed significantly less endothelial nitric oxide synthase protein after exposure to flow than HAECs, nitric oxide levels induced by flow were not significantly different. These results suggest that late outgrowth CB-EPCs are functionally similar to HAECs under flow conditions and are a promising cell source for cardiovascular therapies.
Figures

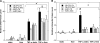
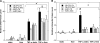

Similar articles
-
Dynamic adhesion of umbilical cord blood endothelial progenitor cells under laminar shear stress.Biophys J. 2010 Dec 1;99(11):3545-54. doi: 10.1016/j.bpj.2010.10.004. Biophys J. 2010. PMID: 21112278 Free PMC article.
-
Unusual transduction response of progenitor-derived and mature endothelial cells exposed to laminar pulsatile shear stress.J Biomech. 2008 Aug 28;41(12):2781-5. doi: 10.1016/j.jbiomech.2008.06.003. Epub 2008 Jul 14. J Biomech. 2008. PMID: 18621377
-
Fluid shear stress induces differentiation of circulating phenotype endothelial progenitor cells.Am J Physiol Cell Physiol. 2012 Sep 15;303(6):C595-606. doi: 10.1152/ajpcell.00133.2012. Epub 2012 Jun 27. Am J Physiol Cell Physiol. 2012. PMID: 22744008
-
Transplantation of umbilical cord blood-derived endothelial progenitor cells: a promising method of therapeutic revascularisation.Eur J Haematol. 2006 Jan;76(1):1-8. doi: 10.1111/j.1600-0609.2005.00579.x. Eur J Haematol. 2006. PMID: 16343265 Review.
-
Therapeutic Potential of Endothelial Colony Forming Cells Derived from Human Umbilical Cord Blood.Curr Stem Cell Res Ther. 2019;14(6):460-465. doi: 10.2174/1574888X14666190214162453. Curr Stem Cell Res Ther. 2019. PMID: 30767752 Review.
Cited by
-
Shear stress regulates late EPC differentiation via mechanosensitive molecule-mediated cytoskeletal rearrangement.PLoS One. 2013 Jul 2;8(7):e67675. doi: 10.1371/journal.pone.0067675. Print 2013. PLoS One. 2013. PMID: 23844056 Free PMC article.
-
Endothelial colony-forming cells show a mature transcriptional response to shear stress.In Vitro Cell Dev Biol Anim. 2012 Jan;48(1):21-9. doi: 10.1007/s11626-011-9470-z. Epub 2011 Nov 20. In Vitro Cell Dev Biol Anim. 2012. PMID: 22101679
-
Late-outgrowth endothelial progenitors from patients with coronary artery disease: endothelialization of confluent stromal cell layers.Acta Biomater. 2014 Feb;10(2):893-900. doi: 10.1016/j.actbio.2013.10.004. Epub 2013 Oct 16. Acta Biomater. 2014. PMID: 24140604 Free PMC article.
-
Application of induced pluripotent stem cells to model smooth muscle cell function in vascular diseases.Curr Opin Biomed Eng. 2017 Mar;1:38-44. doi: 10.1016/j.cobme.2017.02.005. Epub 2017 Mar 22. Curr Opin Biomed Eng. 2017. PMID: 29082353 Free PMC article.
-
Biomechanical effects of flow and coculture on human aortic and cord blood-derived endothelial cells.J Biomech. 2011 Jul 28;44(11):2150-7. doi: 10.1016/j.jbiomech.2011.05.024. Epub 2011 Jun 16. J Biomech. 2011. PMID: 21683362 Free PMC article.
References
-
- Shirota T. He H. Yasui H. Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127. - PubMed
-
- Gong Z. Niklason L.E. Blood vessels engineered from human cells. Trends Cardiovasc Med. 2006;16:153. - PubMed
-
- Shirota T. Yasui H. Matsuda T. Intralumenal tissue-engineered therapeutic stent using endothelial progenitor cell-inoculated hybrid tissue and in vitro performance. Tissue Eng. 2003;9:473. - PubMed
-
- Thompson M.M. Budd J.S. Eady S.L. Underwood M.J. James R.F. Bell P.R. The effect of transluminal endothelial seeding on myointimal hyperplasia following angioplasty. Eur J Vasc Surg. 1994;8:423. - PubMed
-
- Brown M. Wallace C.S. Truskey G.A. Vascular and capillary endothelium. In: Metin Akay., editor. Wiley Encyclopedia of Biomedical Engineering. John Wiley & Sons I; 2006. DOI: 10.1002/9780471740360.ebs0436.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

