VITA-D: cholecalciferol substitution in vitamin D deficient kidney transplant recipients: a randomized, placebo-controlled study to evaluate the post-transplant outcome
- PMID: 19480654
- PMCID: PMC2701431
- DOI: 10.1186/1745-6215-10-36
VITA-D: cholecalciferol substitution in vitamin D deficient kidney transplant recipients: a randomized, placebo-controlled study to evaluate the post-transplant outcome
Abstract
Background: Vitamin D does not only regulate calcium homeostasis but also plays an important role as an immune modulator. It influences the immune system through the induction of immune shifts and regulatory cells resulting in immunologic tolerance. As such, vitamin D is thought to exert beneficial effects within the transplant setting, especially in kidney transplant recipients, considering the high prevalence of vitamin D deficiency in kidney transplant recipients.
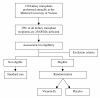
Methods/design: The VITA-D study, a randomized, placebo-controlled, double-blind study with two parallel groups including a total of 200 kidney transplant recipients, is designed to investigate the immunomodulatory and renoprotective effects of cholecalciferol (vitamin D3) within the transplant setting. Kidney transplant recipients found to have vitamin D deficiency defined as 25-hydroxyvitamin D3 < 50 nmol per liter will be randomly assigned to receive either oral cholecalciferol therapy or placebo and will be followed for one year. Cholecalciferol will be administered at a dose of 6800 International Units daily over a time period of one year. The objective is to evaluate the influence of vitamin D3 substitution in vitamin D deficient kidney transplant recipients on the post-transplant outcome. As a primary endpoint glomerular filtration rate calculated with the MDRD formula (modification of diet in renal disease) one year after kidney transplantation will be evaluated. Incidence of acute rejection episodes, and the number and severity of infections (analyzed by means of C-reactive protein) within the first year after transplantation will be monitored as well. As a secondary endpoint the influence of vitamin D3 on bone mineral density within the first year post-transplant will be assessed. Three DXA analyses will be performed, one within the first four weeks post-transplant, one five months and one twelve months after kidney transplantation.
Trial registration: ClinicalTrials.gov NCT00752401.
Figures
References
-
- Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials