In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses
- PMID: 19480655
- PMCID: PMC2702303
- DOI: 10.1186/1471-2229-9-64
In vivo reorganization of the actin cytoskeleton in leaves of Nicotiana tabacum L. transformed with plastin-GFP. Correlation with light-activated chloroplast responses
Abstract
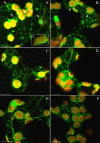
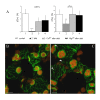
Background: The actin cytoskeleton is involved in the responses of plants to environmental signals. Actin bundles play the role of tracks in chloroplast movements activated by light. Chloroplasts redistribute in response to blue light in the mesophyll cells of Nicotiana tabacum. The aim of this work was to study the relationship between chloroplast responses and the organization of actin cytoskeleton in living tobacco cells. Chloroplast movements were measured photometrically as changes in light transmission through the leaves. The actin cytoskeleton, labeled with plastin-GFP, was visualised by confocal microscopy.
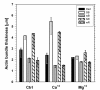
Results: The actin cytoskeleton was affected by strong blue and red light. No blue light specific actin reorganization was detected. EGTA and trifluoperazine strongly inhibited chloroplast responses and disrupted the integrity of the cytoskeleton. This disruption was reversible by Ca(2+) or Mg(2+). Additionally, the effect of trifluoperazine was reversible by light. Wortmannin, an inhibitor of phosphoinositide kinases, potently inhibited chloroplast responses but did not influence the actin cytoskeleton at the same concentration. Also this inhibition was reversed by Ca(2+) and Mg(2+). Magnesium ions were equally or more effective than Ca(2+) in restoring chloroplast motility after treatment with EGTA, trifluoperazine or wortmannin.
Conclusion: The architecture of the actin cytoskeleton in the mesophyll of tobacco is significantly modulated by strong light. This modulation does not affect the direction of chloroplast redistribution in the cell. Calcium ions have multiple functions in the mechanism of the movements. Our results suggest also that Mg(2+) is a regulatory molecule cooperating with Ca(2+) in the signaling pathway of blue light-induced tobacco chloroplast movements.
Figures




Similar articles
-
Actin cytoskeleton in Arabidopsis thaliana under blue and red light.Biol Cell. 2007 May;99(5):251-60. doi: 10.1042/BC20060077. Biol Cell. 2007. PMID: 17253958
-
THRUMIN1 is a light-regulated actin-bundling protein involved in chloroplast motility.Curr Biol. 2011 Jan 11;21(1):59-64. doi: 10.1016/j.cub.2010.11.059. Epub 2010 Dec 23. Curr Biol. 2011. PMID: 21185188
-
Blue light-induced chloroplast reorientations in Lemna trisulca L. (duckweed) are controlled by two separable cellular mechanisms as suggested by different sensitivity to wortmannin.Photochem Photobiol. 2004 Apr;79(4):343-8. doi: 10.1562/le-03-16.1. Photochem Photobiol. 2004. PMID: 15137511
-
Actin-mediated movement of chloroplasts.J Cell Sci. 2018 Jan 29;131(2):jcs210310. doi: 10.1242/jcs.210310. J Cell Sci. 2018. PMID: 29378837 Review.
-
Chloroplast movement.Plant Sci. 2013 Sep;210:177-82. doi: 10.1016/j.plantsci.2013.05.016. Epub 2013 Jun 7. Plant Sci. 2013. PMID: 23849124 Review.
Cited by
-
Evolution of the Cp-Actin-based Motility System of Chloroplasts in Green Plants.Front Plant Sci. 2016 May 3;7:561. doi: 10.3389/fpls.2016.00561. eCollection 2016. Front Plant Sci. 2016. PMID: 27200035 Free PMC article.
-
Chloroplast actin filaments organize meshwork on the photorelocated chloroplasts in the moss Physcomitrella patens.Planta. 2011 Feb;233(2):357-68. doi: 10.1007/s00425-010-1299-2. Epub 2010 Oct 30. Planta. 2011. PMID: 21053010
-
Blue light-dependent changes in loosely bound calcium in Arabidopsis mesophyll cells: an X-ray microanalysis study.J Exp Bot. 2016 Jun;67(13):3953-64. doi: 10.1093/jxb/erw089. Epub 2016 Mar 8. J Exp Bot. 2016. PMID: 26957564 Free PMC article.
-
The very many faces of presenilins and the γ-secretase complex.Protoplasma. 2013 Oct;250(5):997-1011. doi: 10.1007/s00709-013-0494-y. Epub 2013 Mar 16. Protoplasma. 2013. PMID: 23504135 Free PMC article. Review.
-
Plant organellar calcium signalling: an emerging field.J Exp Bot. 2012 Feb;63(4):1525-42. doi: 10.1093/jxb/err394. Epub 2011 Dec 26. J Exp Bot. 2012. PMID: 22200666 Free PMC article. Review.
References
-
- Grolig F. Organelle movements: transport and positioning. In: Hussey PJ, editor. The plant cytoskeleton in cell differentiation and development. Oxford, Blackwell Publishing; 2004. pp. 148–175.
-
- Haupt W, Scheuerlein R. Chloroplast movement. Plant Cell Environ. 1990;13:596–614. doi: 10.1111/j.1365-3040.1990.tb01078.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

