Chapter 11. Subsecond analyses of G-protein coupled-receptor ternary complex dynamics by rapid mix flow cytometry
- PMID: 19480922
- PMCID: PMC4476792
- DOI: 10.1016/S0076-6879(09)05411-1
Chapter 11. Subsecond analyses of G-protein coupled-receptor ternary complex dynamics by rapid mix flow cytometry
Abstract
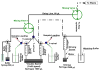
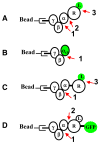
The binding of full and partial agonist ligands (L) to G-protein-coupled receptors (GPCRs) initiates the formation of ternary complexes with G-proteins (LRG complexes). We describe the assembly of detergent-solubilized LRG complexes on beads. Rapid mix flow cytometry is used to analyze the subsecond dynamics of guanine nucleotide-mediated ternary complex disassembly. Ternary complexes were assembled with three formyl peptide receptor constructs (wild type, FPR-Galpha(i2) fusion, and FPR-GFP fusion) and two isotypes of the alpha subunit (alpha(i2) and alpha(i3)) and betagamma dimer (beta(i)(1)gamma(2) and beta(4)gamma(2)). Experimental evidence suggests that thermodynamic stability of ternary complexes depends on subunit isotype. Comparison of assemblies derived from the three constructs of FPR and G-protein heterotrimers composed of the available subunit isotypes demonstrate that the fast step is associated with the separation of receptor and G-protein and that the dissociation of the ligand or of the alpha and betagamma subunits was slower. These results are compatible with a cell activation model involving G-protein conformational changes rather than disassembly of Galphabetagamma heterotrimer.
Figures
Similar articles
-
Rapid-mix flow cytometry measurements of subsecond regulation of G protein-coupled receptor ternary complex dynamics by guanine nucleotides.Anal Biochem. 2007 Dec 1;371(1):10-20. doi: 10.1016/j.ab.2007.08.011. Epub 2007 Aug 14. Anal Biochem. 2007. PMID: 17904091 Free PMC article.
-
Some mechanistic insights into GPCR activation from detergent-solubilized ternary complexes on beads.Adv Protein Chem. 2007;74:95-135. doi: 10.1016/S0065-3233(07)74003-2. Adv Protein Chem. 2007. PMID: 17854656 Review.
-
Ligand-receptor-G-protein molecular assemblies on beads for mechanistic studies and screening by flow cytometry.Mol Pharmacol. 2003 Nov;64(5):1227-38. doi: 10.1124/mol.64.5.1227. Mol Pharmacol. 2003. PMID: 14573773
-
Real-time analysis of G protein-coupled receptor reconstitution in a solubilized system.J Biol Chem. 2001 Jun 22;276(25):22453-60. doi: 10.1074/jbc.M009679200. Epub 2001 Apr 17. J Biol Chem. 2001. PMID: 11309376
-
Signaling through G protein coupled receptors.Plant Signal Behav. 2009 Oct;4(10):942-7. doi: 10.4161/psb.4.10.9530. Epub 2009 Oct 14. Plant Signal Behav. 2009. PMID: 19826234 Free PMC article. Review.
Cited by
-
Use of flow cytometric methods to quantify protein-protein interactions.Curr Protoc Cytom. 2010 Jan;Chapter 13:Unit 13.11.1-15. doi: 10.1002/0471142956.cy1311s51. Curr Protoc Cytom. 2010. PMID: 20069525 Free PMC article.
References
-
- Bennett TA, Key TA, Gurevich VV, Neubig R, Prossnitz ER, Sklar LA. Real-time analysis of G protein-coupled receptor reconstitution in a solubilized system. J Biol Chem. 2001;276:22453–60. - PubMed
-
- Bennett TA, Prossnitz ER, Gurevich VV, Neubig R, Sklar LA. G protein-coupled receptor reconstitution in a solubilized system. Molecular Biology of the Cell. 2000;11:105A–105A. - PubMed
-
- Buranda T, Jones G, Nolan J, Keij J, Lopez GP, Sklar LA. Ligand Receptor Dynamics at Streptavidin Coated Particle Surfaces: A Flow Cytometric and Spectrofluorimetric Study. J Phys Chem B. 1999;103:3399–3410.
-
- Buranda T, Lopez GP, Simons P, Pastuszyn A, Sklar LA. Detection of epitope-tagged proteins in flow cytometry: fluorescence resonance energy transfer-based assays on beads with femtomole resolution. Anal Biochem. 2001;298:151–162. - PubMed
-
- Buranda T, Waller A, Wu Y, Simons PC, Biggs S, Prossnitz ER, Sklar LA. Some mechanistic insights into GPCR activation from detergent-solubilized ternary complexes on beads. Advances in protein chemistry. 2007;74:95–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

