Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation
- PMID: 19481167
- PMCID: PMC2768066
- DOI: 10.1016/j.niox.2009.05.005
Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation
Abstract
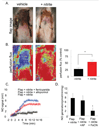
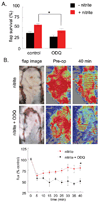
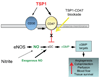
Tissue ischemia and ischemia-reperfusion (I/R) remain sources of cell and tissue death. Inability to restore blood flow and limit reperfusion injury represents a challenge in surgical tissue repair and transplantation. Nitric oxide (NO) is a central regulator of blood flow, reperfusion signaling and angiogenesis. De novo NO synthesis requires oxygen and is limited in ischemic vascular territories. Nitrite (NO(2-)) has been discovered to convert to NO via heme-based reduction during hypoxia, providing a NO synthase independent and oxygen-independent NO source. Furthermore, blockade of the matrix protein thrombospondin-1 (TSP1) or its receptor CD47 has been shown to promote downstream NO signaling via soluble guanylate cyclase (sGC) and cGMP-dependant kinase. We hypothesized that nitrite would provide an ischemic NO source that could be potentiated by TSP1-CD47 blockade enhancing ischemic tissue survival, blood flow and angiogenesis. Both low dose nitrite and direct blockade of TSP1-CD47 interaction using antibodies or gene silencing increased acute blood flow and late tissue survival in ischemic full thickness flaps. Nitrite and TSP1 blockade both enhanced in vitro and in vivo angiogenic responses. The nitrite effect could be abolished by inhibition of sGC and cGMP signaling. Potential therapeutic synergy was tested in a more severe ischemic flap model. We found that combined therapy with nitrite and TSP1-CD47 blockade enhanced flap perfusion, survival and angiogenesis to a greater extent than either agent alone, providing approximately 100% flap survival. These data provide a new therapeutic paradigm for hypoxic NO signaling through enhanced cGMP mediated by TSP1-CD47 blockade and nitrite delivery.
Figures





References
-
- Kerrigan CL, Stotland MA. Ischemia reperfusion injury: a review. Microsurgery. 1993;14:165–175. - PubMed
-
- Howell ST, Seaber AV, Urbaniak JR. Microcirculatory responses to vascular washout following ischemia. Microsurgery. 1989;10:264–268. - PubMed
-
- Kerrigan CL, Zelt RG, Daniel RK. Secondary critical ischemia time of experimental skin flaps. Plast Reconstr Surg. 1984;74:522–526. - PubMed
-
- Pang CY, Forrest CR, Mounsey R. Pharmacologic intervention in ischemia-induced reperfusion injury in the skeletal muscle. Microsurgery. 1993;14:176–182. - PubMed
-
- Riccio M, Pangrazi PP, Campodonico A, Scalise A, Marchesini A, Talevi D, Carboni A, Bertani C, Bertani A, Mazzanti L. Combined use of WEB2170 and HBO therapy can reduce ischemia and reperfusion injury to the skeletal muscle in a rabbit model. Microsurgery. 2007;27:43–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

