Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis
- PMID: 19481529
- PMCID: PMC2744858
- DOI: 10.1016/j.molcel.2009.04.011
Akt and 14-3-3 control a PACS-2 homeostatic switch that integrates membrane traffic with TRAIL-induced apoptosis
Abstract
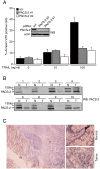
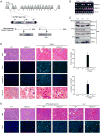
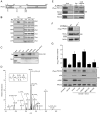
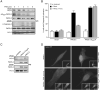
TRAIL selectively kills diseased cells in vivo, spurring interest in this death ligand as a potential therapeutic. However, many cancer cells are resistant to TRAIL, suggesting the mechanism mediating TRAIL-induced apoptosis is complex. Here we identify PACS-2 as an essential TRAIL effector, required for killing tumor cells in vitro and virally infected hepatocytes in vivo. PACS-2 is phosphorylated at Ser437 in vivo, and pharmacologic and genetic studies demonstrate Akt is an in vivo Ser437 kinase. Akt cooperates with 14-3-3 to regulate the homeostatic and apoptotic properties of PACS-2 that mediate TRAIL action. Phosphorylated Ser437 binds 14-3-3 with high affinity, which represses PACS-2 apoptotic activity and is required for PACS-2 to mediate trafficking of membrane cargo. TRAIL triggers dephosphorylation of Ser437, reprogramming PACS-2 to promote apoptosis. Together, these studies identify the phosphorylation state of PACS-2 Ser437 as a molecular switch that integrates cellular homeostasis with TRAIL-induced apoptosis.
Figures


References
-
- Anderson GR, Brenner BM, Swede H, Chen N, Henry WM, Conroy JM, Karpenko MJ, Issa JP, Bartos JD, Brunelle JK, et al. Intrachromosomal genomic instability in human sporadic colorectal cancer measured by genome-wide allelotyping and inter-(simple sequence repeat) PCR. Cancer Res. 2001;61:8274–8283. - PubMed
-
- Atkins KM, Thomas L, Youker RT, Harriff MJ, Pissani F, You H, Thomas G. HIV-1 Nef Binds PACS-2 to Assemble a Multikinase Cascade That Triggers Major Histocompatibility Complex Class I (MHC-I) Down-regulation: ANALYSIS USING SHORT INTERFERING RNA AND KNOCK-OUT MICE. J Biol Chem. 2008;283:11772–11784. - PMC - PubMed
-
- Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, Pandey SK, Nwokorie C, Onwudiwe N, Srivastava RK. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI049793/AI/NIAID NIH HHS/United States
- AI49793/AI/NIAID NIH HHS/United States
- T32 AI07472/AI/NIAID NIH HHS/United States
- RR0241/RR/NCRR NIH HHS/United States
- P50CA128613/CA/NCI NIH HHS/United States
- T32 DK007680/DK/NIDDK NIH HHS/United States
- T32 AI007472/AI/NIAID NIH HHS/United States
- T32 GM071338/GM/NIGMS NIH HHS/United States
- P01CA116676/CA/NCI NIH HHS/United States
- P50 CA128613/CA/NCI NIH HHS/United States
- UL1 TR000128/TR/NCATS NIH HHS/United States
- P50 CA097186/CA/NCI NIH HHS/United States
- F32 DK076343/DK/NIDDK NIH HHS/United States
- R01 DK037274/DK/NIDDK NIH HHS/United States
- P50CA97186/CA/NCI NIH HHS/United States
- P01 CA116676/CA/NCI NIH HHS/United States
- DK3724/DK/NIDDK NIH HHS/United States
- DK076343/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

