30 years of dynorphins--new insights on their functions in neuropsychiatric diseases
- PMID: 19481570
- PMCID: PMC2872771
- DOI: 10.1016/j.pharmthera.2009.05.006
30 years of dynorphins--new insights on their functions in neuropsychiatric diseases
Abstract
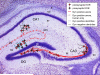
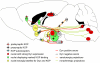
Since the first description of their opioid properties three decades ago, dynorphins have increasingly been thought to play a regulatory role in numerous functional pathways of the brain. Dynorphins are members of the opioid peptide family and preferentially bind to kappa opioid receptors. In line with their localization in the hippocampus, amygdala, hypothalamus, striatum and spinal cord, their functions are related to learning and memory, emotional control, stress response and pain. Pathophysiological mechanisms that may involve dynorphins/kappa opioid receptors include epilepsy, addiction, depression and schizophrenia. Most of these functions were proposed in the 1980s and 1990s following histochemical, pharmacological and electrophysiological experiments using kappa receptor-specific or general opioid receptor agonists and antagonists in animal models. However, at that time, we had little information on the functional relevance of endogenous dynorphins. This was mainly due to the complexity of the opioid system. Besides actions of peptides from all three classical opioid precursors (proenkephalin, prodynorphin, proopiomelanocortin) on the three classical opioid receptors (delta, mu and kappa), dynorphins were also shown to exert non-opioid effects mainly through direct effects on NMDA receptors. Moreover, discrepancies between the distribution of opioid receptor binding sites and dynorphin immunoreactivity contributed to the difficulties in interpretation. In recent years, the generation of prodynorphin- and opioid receptor-deficient mice has provided the tools to investigate open questions on network effects of endogenous dynorphins. This article examines the physiological, pathophysiological and pharmacological implications of dynorphins in the light of new insights in part obtained from genetically modified animals.
Figures

References
-
- Aradi I, Santhakumar V, Chen K, Soltesz I. Postsynaptic effects of GABAergic synaptic diversity: regulation of neuronal excitability by changes in IPSC variance. Neuropharmacology. 2002;43:511–522. - PubMed
-
- Bakalkin G, Yakovleva T, Terenius L. Prodynorphin gene expression relates to NF-kappa B factors. Brain Res Mol Brain Res. 1994;24:301–312. - PubMed
-
- Bakshi R, Faden AI. Competitive and non-competitive NMDA antagonists limit dynorphin A-induced rat hindlimb paralysis. Brain Res. 1990;507:1–5. - PubMed
-
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

