The microanatomy of B cell activation
- PMID: 19481917
- PMCID: PMC3736860
- DOI: 10.1016/j.coi.2009.05.006
The microanatomy of B cell activation
Abstract
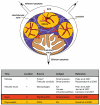
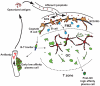
The logistic problem of B cell antigen encounter in the lymph node has recently been studied by dynamic imaging using two-photon microscopy. These studies combined with the early studies of antigen transport have yielded a more complete picture of the orchestration of B cell activation in vivo. Here we summarize the recent advances and focus on the specialized macrophages that are critical to this process and the role of B cells themselves as antigen transporting cells.
Figures
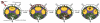
References
-
- Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 1997;156:11–24. - PubMed
-
- Fossum S. The architecture of rat lymph nodes. IV. Distribution of ferritin and colloidal carbon in the draining lymph nodes after foot-pad injection. Scand J Immunol. 1980;12:433–441. - PubMed
-
- Sainte-Marie G, Peng FS, Belisle C. Overall architecture and pattern of lymph flow in the rat lymph node. Am J Anat. 1982;164:275–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

