Chasing the treasures of the sea - bacterial marine natural products
- PMID: 19481972
- PMCID: PMC2695832
- DOI: 10.1016/j.mib.2009.05.002
Chasing the treasures of the sea - bacterial marine natural products
Abstract
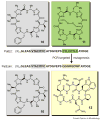
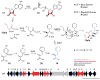
Bacterial marine natural products are an important source of novel lead structures for drug discovery. The cytotoxic properties of many of these secondary metabolites are of particular interest for the development of new anticancer agents. Tremendous advances in marine molecular biology, genome sequencing, and bioinformatics have paved the way to fully exploit the biomedical potential of marine bacterial products. In addition, unique biosynthetic enzymes discovered from bacteria from the sea have begun to emerge as powerful biocatalysts in medicinal chemistry and total synthesis. The increasingly interdisciplinary field of marine natural product chemistry thus strongly impacts future developments in medicine, chemistry, and biology.
Figures


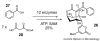
References
-
- Newmann DJ, Cragg GM. Natural Products as Sources of New Drugs over the Last 25 Years. J Nat Prod. 2007;70:461–477. - PubMed
-
- Blunt JW, Copp BR, Hu W-P, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2008;25:35–94. - PubMed
-
- Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007;68:954–979. - PubMed
-
- Fenical W, Jensen PR. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–673. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

