TC-5619: an alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia
- PMID: 19482012
- PMCID: PMC3005247
- DOI: 10.1016/j.bcp.2009.05.030
TC-5619: an alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia
Abstract
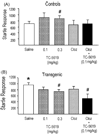
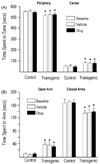
A growing body of evidence suggests that the alpha7 neuronal nicotinic receptor (NNR) subtype is an important target for the development of novel therapies to treat schizophrenia, offering the possibility to address not only the positive but also the cognitive and negative symptoms associated with the disease. In order to probe the relationship of alpha7 function to relevant behavioral correlates we employed TC-5619, a novel selective agonist for the alpha7 NNR subtype. TC-5619 binds with very high affinity to the alpha7 subtype and is a potent full agonist. TC-5619 has little or no activity at other nicotinic receptors, including the alpha4beta2, ganglionic (alpha3beta4) and muscle subtypes. The transgenic th(tk-)/th(tk-) mouse model that reflects many of the developmental, anatomical, and multi-transmitter biochemical aspects of schizophrenia was used to assess the antipsychotic effects of TC-5619. In these mice TC-5619 acted both alone and synergistically with the antipsychotic clozapine to correct impaired pre-pulse inhibition (PPI) and social behavior which model positive and negative symptoms, respectively. Antipsychotic and cognitive effects of TC-5619 were also assessed in rats. Similar to the results in the transgenic mice, TC-5619 significantly reversed apomorphine-induced PPI deficits. In a novel object recognition paradigm in rats TC-5619 demonstrated long-lasting enhancement of memory over a wide dose range. These results suggest that alpha7-selective agonists such as TC-5619, either alone or in combination with antipsychotics, could offer a new approach to treating the constellation of symptoms associated with schizophrenia, including cognitive dysfunction.
Figures





Similar articles
-
SSR180711, a novel selective alpha7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia.Neuropsychopharmacology. 2007 Jan;32(1):17-34. doi: 10.1038/sj.npp.1301188. Epub 2006 Aug 23. Neuropsychopharmacology. 2007. PMID: 16936709
-
The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide improves working and recognition memory in rodents.J Pharmacol Exp Ther. 2007 May;321(2):716-25. doi: 10.1124/jpet.106.118976. Epub 2007 Feb 16. J Pharmacol Exp Ther. 2007. PMID: 17308038
-
α7 neuronal nicotinic receptor agonist (TC-7020) reverses increased striatal dopamine release during acoustic PPI testing in a transgenic mouse model of schizophrenia.Schizophr Res. 2012 Apr;136(1-3):82-7. doi: 10.1016/j.schres.2012.01.005. Epub 2012 Jan 30. Schizophr Res. 2012. PMID: 22285656
-
Alpha7 neuronal nicotinic receptors as targets for novel therapies to treat multiple domains of schizophrenia.Curr Pharm Biotechnol. 2011 Mar 1;12(3):437-48. doi: 10.2174/138920111794480589. Curr Pharm Biotechnol. 2011. PMID: 21133847 Review.
-
Selective nicotinic acetylcholine receptor agonists: potential therapies for neuropsychiatric disorders with cognitive dysfunction.Curr Opin Investig Drugs. 2008 Jan;9(1):47-56. Curr Opin Investig Drugs. 2008. PMID: 18183531 Review.
Cited by
-
Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology.Int J Mol Sci. 2012;13(2):2219-2238. doi: 10.3390/ijms13022219. Epub 2012 Feb 17. Int J Mol Sci. 2012. PMID: 22408449 Free PMC article. Review.
-
Synthesis, Pharmacological Characterization, and Structure-Activity Relationships of Noncanonical Selective Agonists for α7 nAChRs.J Med Chem. 2019 Nov 27;62(22):10376-10390. doi: 10.1021/acs.jmedchem.9b01467. Epub 2019 Nov 19. J Med Chem. 2019. PMID: 31675224 Free PMC article.
-
Reduced CHRNA7 expression in C3H mice is associated with increases in hippocampal parvalbumin and glutamate decarboxylase-67 (GAD67) as well as altered levels of GABA(A) receptor subunits.Neuroscience. 2014 Jul 25;273:52-64. doi: 10.1016/j.neuroscience.2014.05.004. Epub 2014 May 13. Neuroscience. 2014. PMID: 24836856 Free PMC article.
-
Targeting the Cholinergic System to Develop a Novel Therapy for Huntington's Disease.J Huntingtons Dis. 2016 Dec 15;5(4):333-342. doi: 10.3233/JHD-160200. J Huntingtons Dis. 2016. PMID: 27983560 Free PMC article. Review.
-
Characterizing the binding of TC-5619 and encenicline on the alpha7 nicotinic acetylcholine receptor using PET imaging in the pig.Front Neuroimaging. 2024 Mar 27;3:1358221. doi: 10.3389/fnimg.2024.1358221. eCollection 2024. Front Neuroimaging. 2024. PMID: 38601007 Free PMC article.
References
-
- Wong AH, Van Tol HH. Schizophrenia: from phenomenology to neurobiology. Neurosci Biobehav Rev. 2003;27:269–306. - PubMed
-
- Barnes T, Drake R. Pharmacological strategies for relapse prevention in schizophrenia. Psychiatry (England) 2007;6:351–356.
-
- Holden C. Neuroscience. Deconstructing schizophrenia. Science. 2003;299:333–335. - PubMed
-
- Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28:2184–2191. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

