RNA chaperones stimulate formation and yield of the U3 snoRNA-Pre-rRNA duplexes needed for eukaryotic ribosome biogenesis
- PMID: 19482034
- PMCID: PMC2881153
- DOI: 10.1016/j.jmb.2009.05.072
RNA chaperones stimulate formation and yield of the U3 snoRNA-Pre-rRNA duplexes needed for eukaryotic ribosome biogenesis
Abstract
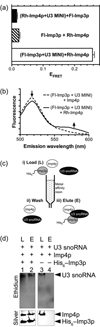
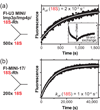
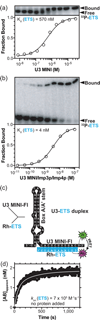
Short duplexes between the U3 small nucleolar RNA and the precursor ribosomal RNA must form quickly and with high yield to satisfy the high demand for ribosome synthesis in rapidly growing eukaryotic cells. These interactions, designated the U3-ETS (external transcribed spacer) and U3-18S duplexes, are essential to initiate the processing of small subunit ribosomal RNA. Previously, we showed that duplexes corresponding to those in Saccharomyces cerevisiae are only observed in vitro after addition of one of two proteins: Imp3p or Imp4p. Here, we used fluorescence-based and other in vitro assays to determine whether these proteins possess RNA chaperone activities and to assess whether these activities are sufficient to satisfy the duplex yield and rate requirements expected in vivo. Assembly of both proteins with the U3 small nucleolar RNA into a chaperone complex destabilizes a U3 stem structure, apparently to expose its 18S base-pairing site. As a result, the chaperone complex accelerates formation of the U3-18S duplex from an undetectable rate to one comparable with the intrinsic rate observed for hybridizing short duplexes. The chaperone complex also stabilizes the U3-ETS duplex by 2.7 kcal/mol. These chaperone activities provide high U3-ETS duplex yield and rapid U3-18S duplex formation over a broad concentration range to help ensure that the U3-precursor ribosomal RNA interactions limit neither ribosome biogenesis nor rapid cell growth. The thermodynamic and kinetic framework used is general and thus suitable for investigating the mechanism of action of other RNA chaperones.
Figures







Similar articles
-
The small subunit processome in ribosome biogenesis—progress and prospects.Wiley Interdiscip Rev RNA. 2011 Jan-Feb;2(1):1-21. doi: 10.1002/wrna.57. Wiley Interdiscip Rev RNA. 2011. PMID: 21318072 Free PMC article. Review.
-
Imp3 unfolds stem structures in pre-rRNA and U3 snoRNA to form a duplex essential for small subunit processing.RNA. 2013 Oct;19(10):1372-83. doi: 10.1261/rna.039511.113. Epub 2013 Aug 26. RNA. 2013. PMID: 23980203 Free PMC article.
-
Imp3p and Imp4p mediate formation of essential U3-precursor rRNA (pre-rRNA) duplexes, possibly to recruit the small subunit processome to the pre-rRNA.Proc Natl Acad Sci U S A. 2004 Oct 26;101(43):15301-6. doi: 10.1073/pnas.0406819101. Epub 2004 Oct 15. Proc Natl Acad Sci U S A. 2004. PMID: 15489263 Free PMC article.
-
UtpA and UtpB chaperone nascent pre-ribosomal RNA and U3 snoRNA to initiate eukaryotic ribosome assembly.Nat Commun. 2016 Jun 29;7:12090. doi: 10.1038/ncomms12090. Nat Commun. 2016. PMID: 27354316 Free PMC article.
-
RNA folding and functions of RNA helicases in ribosome biogenesis.RNA Biol. 2022 Jan;19(1):781-810. doi: 10.1080/15476286.2022.2079890. RNA Biol. 2022. PMID: 35678541 Free PMC article. Review.
Cited by
-
Elucidation of the assembly events required for the recruitment of Utp20, Imp4 and Bms1 onto nascent pre-ribosomes.Nucleic Acids Res. 2011 Oct;39(18):8105-21. doi: 10.1093/nar/gkr508. Epub 2011 Jun 30. Nucleic Acids Res. 2011. PMID: 21724601 Free PMC article.
-
Implication of the box C/D snoRNP assembly factor Rsa1p in U3 snoRNP assembly.Nucleic Acids Res. 2017 Jul 7;45(12):7455-7473. doi: 10.1093/nar/gkx424. Nucleic Acids Res. 2017. PMID: 28505348 Free PMC article.
-
The small subunit processome in ribosome biogenesis—progress and prospects.Wiley Interdiscip Rev RNA. 2011 Jan-Feb;2(1):1-21. doi: 10.1002/wrna.57. Wiley Interdiscip Rev RNA. 2011. PMID: 21318072 Free PMC article. Review.
-
Purification, crystallization and preliminary X-ray diffraction analysis of Imp3 in complex with an Mpp10 peptide involved in yeast ribosome biogenesis.Acta Crystallogr F Struct Biol Commun. 2014 Jul;70(Pt 7):918-21. doi: 10.1107/S2053230X14010905. Epub 2014 Jun 18. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 25005089 Free PMC article.
-
hUTP24 is essential for processing of the human rRNA precursor at site A1, but not at site A0.RNA Biol. 2015;12(9):1010-29. doi: 10.1080/15476286.2015.1073437. RNA Biol. 2015. PMID: 26237581 Free PMC article.
References
-
- Herschlag D. RNA chaperones and the RNA folding problem. J Biol Chem. 1995;270:20871–20874. - PubMed
-
- Granneman S, Baserga SJ. Ribosome biogenesis: of knobs and RNA processing. Exp Cell Res. 2004;296:43–50. - PubMed
-
- Oskarsson T, Trumpp A. The Myc trilogy: lord of RNA polymerases. Nature Cell Biology. 2005;7:215–217. [comment] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

