Functional roles of short sequence motifs in the endocytosis of membrane receptors
- PMID: 19482617
- PMCID: PMC2751658
- DOI: 10.2741/3599
Functional roles of short sequence motifs in the endocytosis of membrane receptors
Abstract
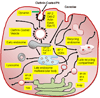
Internalization and trafficking of cell-surface membrane receptors and proteins into subcellular compartments is mediated by specific short-sequence signal motifs, which are usually located within the cytoplasmic domains of these receptor and protein molecules. The signals usually consist of short linear amino acid sequences, which are recognized by adaptor coat proteins along the endocytic and sorting pathways. The complex arrays of signals and recognition proteins ensure the dynamic movement, accurate trafficking, and designated distribution of transmembrane receptors and ligands into intracellular compartments, particularly to the endosomal-lysosomal system. This review summarizes the new information and concepts, integrating them with the current and established views of endocytosis, intracellular trafficking, and sorting of membrane receptors and proteins. Particular emphasis has been given to the functional roles of short-sequence signal motifs responsible for the itinerary and destination of membrane receptors and proteins moving into the subcellular compartments. The specific characteristics and functions of short-sequence motifs, including various tyrosine-based, dileucine-type, and other short-sequence signals in the trafficking and sorting of membrane receptors and membrane proteins are presented and discussed.
Figures



Similar articles
-
Ligand-mediated endocytosis and intracellular sequestration of guanylyl cyclase/natriuretic peptide receptors: role of GDAY motif.Mol Cell Biochem. 2010 Jan;334(1-2):81-98. doi: 10.1007/s11010-009-0332-x. Epub 2009 Nov 26. Mol Cell Biochem. 2010. PMID: 19941037 Free PMC article.
-
Small peptide recognition sequence for intracellular sorting.Curr Opin Biotechnol. 2010 Oct;21(5):611-20. doi: 10.1016/j.copbio.2010.08.007. Curr Opin Biotechnol. 2010. PMID: 20817434 Free PMC article. Review.
-
Signals for sorting of transmembrane proteins to endosomes and lysosomes.Annu Rev Biochem. 2003;72:395-447. doi: 10.1146/annurev.biochem.72.121801.161800. Epub 2003 Mar 6. Annu Rev Biochem. 2003. PMID: 12651740 Review.
-
Sorting motifs in receptor trafficking.Adv Drug Deliv Rev. 2003 Nov 14;55(11):1405-19. doi: 10.1016/j.addr.2003.07.003. Adv Drug Deliv Rev. 2003. PMID: 14597138 Review.
-
Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment.J Cell Biol. 1994 Jul;126(2):317-30. doi: 10.1083/jcb.126.2.317. J Cell Biol. 1994. PMID: 8034737 Free PMC article.
Cited by
-
Ligand-mediated endocytosis and intracellular sequestration of guanylyl cyclase/natriuretic peptide receptors: role of GDAY motif.Mol Cell Biochem. 2010 Jan;334(1-2):81-98. doi: 10.1007/s11010-009-0332-x. Epub 2009 Nov 26. Mol Cell Biochem. 2010. PMID: 19941037 Free PMC article.
-
Structural Domains of the Herpes Simplex Type 1 gD Protein that Restrict HIV-1 Particle Infectivity.J Virol. 2021 Mar 25;95(8):e02355-20. doi: 10.1128/JVI.02355-20. Epub 2021 Feb 3. J Virol. 2021. PMID: 33536165 Free PMC article.
-
Functional analysis of a breast cancer-associated mutation in the intracellular domain of the metalloprotease ADAM12.PLoS One. 2012;7(5):e37628. doi: 10.1371/journal.pone.0037628. Epub 2012 May 25. PLoS One. 2012. PMID: 22662180 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus (KSHV) gB dictates a low-pH endocytotic entry pathway as revealed by a dual-fluorescent virus system and a rhesus monkey rhadinovirus expressing KSHV gB.PLoS Pathog. 2025 Jan 16;21(1):e1012846. doi: 10.1371/journal.ppat.1012846. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39820197 Free PMC article.
-
Insights Into Protein S-Palmitoylation in Synaptic Plasticity and Neurological Disorders: Potential and Limitations of Methods for Detection and Analysis.Front Mol Neurosci. 2018 May 29;11:175. doi: 10.3389/fnmol.2018.00175. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29910712 Free PMC article. Review.
References
-
- Goldstein JL, Anderson RG, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979;279(5715):679–685. - PubMed
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
-
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

