Calpain and the glutamatergic synapse
- PMID: 19482714
- PMCID: PMC2895305
- DOI: 10.2741/s38
Calpain and the glutamatergic synapse
Abstract
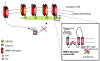
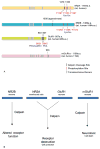
Calpain is a ubiquitous protease found in different tissue types and in many organisms including mammals. It generally does not destroy its large variety of substrates, but more commonly disrupts their function. In neurons, many of its substrates become dysregulated as a result of cleavage of their regulatory domain by this protease, leading to altered signaling between cells. In glutamatergic synaptic transmission, direct targets of calpain include all of the major glutamate receptors: NMDA receptors, AMPA receptors and mGluR. By cleaving these receptors and associated intracellular proteins, calpain may regulate the physiology at glutamatergic synapses. As a result, calpain-mediated cleavage in neurons might not only be involved in pathological events like excitotoxicity, but may also have neuroprotective effects and roles in physiological synaptic transmission.
Figures

References
-
- Collingridge Graham L, Isaac John TR, Wang Yu Tian. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. - PubMed
-
- Lynch David R, Guttmann Rodney P. Excitotoxicity: Perspectives based on N-methyl-D-aspartate receptor subtypes. J Pharmacol Exp Ther. 2002;3:717–723. - PubMed
-
- Dingledine Raymond, Borges Karin, Bowie Derek, Traynelis Stephen F. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–62. - PubMed
-
- Obrenovitch Tihomir P, Urenjak Jutta. Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Prog Neurobiol. 1997;51:39–87. - PubMed
-
- Ottersen Ole P, Landsend Alf S. Organization of glutamate receptors at the synapse. Eur J Neurosci. 1997;9:2219–2224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

