The role of annexin 1 in drought stress in Arabidopsis
- PMID: 19482919
- PMCID: PMC2705051
- DOI: 10.1104/pp.109.135228
The role of annexin 1 in drought stress in Arabidopsis
Abstract
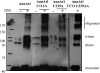
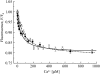
Annexins act as targets of calcium signals in eukaryotic cells, and recent results suggest that they play an important role in plant stress responses. We found that in Arabidopsis (Arabidopsis thaliana), AnnAt1 (for annexin 1) mRNA levels were up-regulated in leaves by most of the stress treatments applied. Plants overexpressing AnnAt1 protein were more drought tolerant and knockout plants were more drought sensitive than ecotype Columbia plants. We also observed that hydrogen peroxide accumulation in guard cells was reduced in overexpressing plants and increased in knockout plants both before and after treatment with abscisic acid. Oxidative protection resulting from AnnAt1 overexpression could be due to the low level of intrinsic peroxidase activity exhibited by this protein in vitro, previously linked to a conserved histidine residue found in a peroxidase-like motif. However, analyses of a mutant H40A AnnAt1 protein in a bacterial complementation test and in peroxidase activity assays indicate that this residue is not critical to the ability of AnnAt1 to confer oxidative protection. To further examine the mechanism(s) linking AnnAt1 expression to stress resistance, we analyzed the reactive S3 cluster to determine if it plays a role in AnnAt1 oligomerization and/or is the site for posttranslational modification. We found that the two cysteine residues in this cluster do not form intramolecular or intermolecular bonds but are highly susceptible to oxidation-driven S-glutathionylation, which decreases the Ca(2+) affinity of AnnAt1 in vitro. Moreover, S-glutathionylation of AnnAt1 occurs in planta after abscisic acid treatment, which suggests that this modification could be important in regulating the cellular function of AnnAt1 during stress responses.
Figures





Comment in
-
Is annexin 1 a multifunctional protein during stress responses?Plant Signal Behav. 2010 Mar;5(3):303-7. doi: 10.4161/psb.5.3.10835. Epub 2010 Mar 2. Plant Signal Behav. 2010. PMID: 20215861 Free PMC article.
Similar articles
-
Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses.Plant Cell Physiol. 2010 Sep;51(9):1499-514. doi: 10.1093/pcp/pcq111. Epub 2010 Jul 23. Plant Cell Physiol. 2010. PMID: 20656895
-
Peroxidase activity of annexin 1 from Arabidopsis thaliana.Biochem Biophys Res Commun. 2005 Oct 28;336(3):868-75. doi: 10.1016/j.bbrc.2005.08.181. Biochem Biophys Res Commun. 2005. PMID: 16153598
-
Potential role of annexin AnnAt1 from Arabidopsis thaliana in pH-mediated cellular response to environmental stimuli.Plant Cell Physiol. 2007 Jun;48(6):792-803. doi: 10.1093/pcp/pcm046. Epub 2007 Apr 22. Plant Cell Physiol. 2007. PMID: 17452342
-
Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis.Plant Cell. 2004 Jun;16(6):1378-91. doi: 10.1105/tpc.021683. Epub 2004 May 25. Plant Cell. 2004. PMID: 15161963 Free PMC article.
-
[Participation of annexin At1 in plant response to abiotic stress].Postepy Biochem. 2007;53(2):154-8. Postepy Biochem. 2007. PMID: 17969875 Review. Polish.
Cited by
-
The rice annexin gene OsAnn5 is involved in cold stress tolerance at the seedling stage.Plant Direct. 2023 Nov 6;7(11):e539. doi: 10.1002/pld3.539. eCollection 2023 Nov. Plant Direct. 2023. PMID: 37942234 Free PMC article.
-
S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells.Cell Cycle. 2015;14(4):502-9. doi: 10.1080/15384101.2014.995495. Cell Cycle. 2015. PMID: 25565331 Free PMC article.
-
Proteomic and functional analyses of Nelumbo nucifera annexins involved in seed thermotolerance and germination vigor.Planta. 2012 Jun;235(6):1271-88. doi: 10.1007/s00425-011-1573-y. Epub 2011 Dec 14. Planta. 2012. PMID: 22167260
-
SpUSP, an annexin-interacting universal stress protein, enhances drought tolerance in tomato.J Exp Bot. 2012 Sep;63(15):5593-606. doi: 10.1093/jxb/ers220. Epub 2012 Aug 21. J Exp Bot. 2012. PMID: 22915741 Free PMC article.
-
Pearl millet response to drought: A review.Front Plant Sci. 2023 Feb 10;14:1059574. doi: 10.3389/fpls.2023.1059574. eCollection 2023. Front Plant Sci. 2023. PMID: 36844091 Free PMC article.
References
-
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55 373–399 - PubMed
-
- Aracena P, Sanchez G, Donoso P, Hamilton SL, Hildago C (2003) S-Glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J Biol Chem 278 42927–42935 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

