Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases
- PMID: 19482945
- PMCID: PMC2701047
- DOI: 10.1073/pnas.0904689106
Induced pluripotent stem cells offer new approach to therapy in thalassemia and sickle cell anemia and option in prenatal diagnosis in genetic diseases
Abstract
The innovation of reprogramming somatic cells to induced pluripotent stem cells provides a possible new approach to treat beta-thalassemia and other genetic diseases such as sickle cell anemia. Induced pluripotent stem (iPS) cells can be made from these patients' somatic cells and the mutation in the beta-globin gene corrected by gene targeting, and the cells differentiated into hematopoietic cells to be returned to the patient. In this study, we reprogrammed the skin fibroblasts of a patient with homozygous beta(0) thalassemia into iPS cells, and showed that the iPS cells could be differentiated into hematopoietic cells that synthesized hemoglobin. Prenatal diagnosis and selective abortion have been effective in decreasing the number of beta-thalassemia births in some countries that have instituted carrier screening and genetic counseling. To make use of the cells from the amniotic fluid or chorionic villus sampling that are used for prenatal diagnosis, we also showed that these cells could be reprogrammed into iPS cells. This raises the possibility of providing a new option following prenatal diagnosis of a fetus affected by a severe illness. Currently, the parents would choose either to terminate the pregnancy or continue it and take care of the sick child after birth. The cells for prenatal diagnosis can be converted into iPS cells for treatment in the perinatal periods. Early treatment has the advantage of requiring much fewer cells than adult treatment, and can also prevent organ damage in those diseases in which damage can begin in utero or at an early age.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
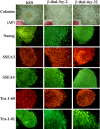



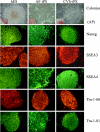

References
-
- Weatherall D, Clegg J. The Thalassaemia Syndromes. 2nd Ed. New York: Wiley-Blackwell; 2001.
-
- Giardini C, Lucarelli G. Bone marrow transplantation for beta-thalassemia. Hematol Oncol Clin North Am. 1999;13:1059–1064. - PubMed
-
- Locatelli F, et al. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood. 2003;101:2137–2143. - PubMed
-
- Rivella S, May C, Chadburn A, Riviere I, Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human beta-globin gene transfer. Blood. 2003;101:2932–2939. - PubMed
-
- Pawliuk R, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294(5550):2368–2371. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

