Sex differences in the expression of hepatic drug metabolizing enzymes
- PMID: 19483103
- PMCID: PMC2713118
- DOI: 10.1124/mol.109.056705
Sex differences in the expression of hepatic drug metabolizing enzymes
Abstract
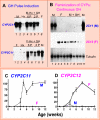
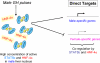
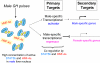
Sex differences in pharmacokinetics and pharmacodynamics characterize many drugs and contribute to individual differences in drug efficacy and toxicity. Sex-based differences in drug metabolism are the primary cause of sex-dependent pharmacokinetics and reflect underlying sex differences in the expression of hepatic enzymes active in the metabolism of drugs, steroids, fatty acids and environmental chemicals, including cytochromes P450 (P450s), sulfotransferases, glutathione transferases, and UDP-glucuronosyltransferases. Studies in the rat and mouse liver models have identified more than 1000 genes whose expression is sex-dependent; together, these genes impart substantial sexual dimorphism to liver metabolic function and pathophysiology. Sex differences in drug metabolism and pharmacokinetics also occur in humans and are due in part to the female-predominant expression of CYP3A4, the most important P450 catalyst of drug metabolism in human liver. The sexually dimorphic expression of P450s and other liver-expressed genes is regulated by the temporal pattern of plasma growth hormone (GH) release by the pituitary gland, which shows significant sex differences. These differences are most pronounced in rats and mice, where plasma GH profiles are highly pulsatile (intermittent) in male animals versus more frequent (nearly continuous) in female animals. This review discusses key features of the cell signaling and molecular regulatory mechanisms by which these sex-dependent plasma GH patterns impart sex specificity to the liver. Moreover, the essential role proposed for the GH-activated transcription factor signal transducer and activator of transcription (STAT) 5b, and for hepatic nuclear factor (HNF) 4alpha, as mediators of the sex-dependent effects of GH on the liver, is evaluated. Together, these studies of the cellular, molecular, and gene regulatory mechanisms that underlie sex-based differences in liver gene expression have provided novel insights into the physiological regulation of both xenobiotic and endobiotic metabolism.
Figures
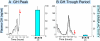

Similar articles
-
Role of hepatocyte nuclear factors in growth hormone-regulated, sexually dimorphic expression of liver cytochromes P450.Growth Factors. 2004 Jun;22(2):79-88. doi: 10.1080/08977190410001715172. Growth Factors. 2004. PMID: 15253383 Review.
-
Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic Acid microarray analysis.Mol Endocrinol. 2004 Mar;18(3):747-60. doi: 10.1210/me.2003-0138. Epub 2003 Dec 18. Mol Endocrinol. 2004. PMID: 14684848
-
Growth hormone regulation of sex-dependent liver gene expression.Mol Endocrinol. 2006 Nov;20(11):2613-29. doi: 10.1210/me.2006-0007. Epub 2006 Mar 16. Mol Endocrinol. 2006. PMID: 16543404 Review.
-
Codependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4alpha.Mol Endocrinol. 2006 Mar;20(3):647-60. doi: 10.1210/me.2005-0328. Epub 2005 Oct 20. Mol Endocrinol. 2006. PMID: 16239260
-
Regulation of liver-specific steroid metabolizing cytochromes P450: cholesterol 7α-hydroxylase, bile acid 6β-hydroxylase, and growth hormone-responsive steroid hormone hydroxylases.J Steroid Biochem Mol Biol. 1992 Dec;43(8):1055-72. doi: 10.1016/0960-0760(92)90333-E. J Steroid Biochem Mol Biol. 1992. PMID: 22217850 Review.
Cited by
-
Corticosteroid-Binding Globulin (SERPINA6) Consolidates Sexual Dimorphism of Adult Rat Liver.Endocrinology. 2023 Nov 20;165(1):bqad179. doi: 10.1210/endocr/bqad179. Endocrinology. 2023. PMID: 38015819 Free PMC article.
-
Ten simple rules in biomedical engineering to improve healthcare equity.PLoS Comput Biol. 2022 Oct 13;18(10):e1010525. doi: 10.1371/journal.pcbi.1010525. eCollection 2022 Oct. PLoS Comput Biol. 2022. PMID: 36227840 Free PMC article. No abstract available.
-
Hepatotoxicity with High-Dose Green Tea Extract: Effect of Catechol-O-Methyltransferase and Uridine 5'-Diphospho-glucuronosyltransferase 1A4 Genotypes.J Diet Suppl. 2023;20(6):850-869. doi: 10.1080/19390211.2022.2128501. Epub 2022 Sep 30. J Diet Suppl. 2023. PMID: 36178169 Free PMC article. Clinical Trial.
-
Sex differences in the brain: a whole body perspective.Biol Sex Differ. 2015 Aug 15;6:15. doi: 10.1186/s13293-015-0032-z. eCollection 2015. Biol Sex Differ. 2015. PMID: 26279833 Free PMC article. Review.
-
Male-specific hepatic Bcl6: growth hormone-induced block of transcription elongation in females and binding to target genes inversely coordinated with STAT5.Mol Endocrinol. 2009 Nov;23(11):1914-26. doi: 10.1210/me.2009-0242. Epub 2009 Oct 1. Mol Endocrinol. 2009. PMID: 19797429 Free PMC article.
References
-
- Agrawal AK and Shapiro BH (2000) Differential expression of gender-dependent hepatic isoforms of cytochrome P-450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat. J Pharmacol Exp Ther 292 228-237. - PubMed
-
- Agrawal AK and Shapiro BH (2001) Intrinsic signals in the sexually dimorphic circulating growth hormone profiles of the rat. Mol Cell Endocrinol 173 167-181. - PubMed
-
- Amador-Noguez D, Zimmerman J, Venable S, and Darlington G (2005) Gender-specific alterations in gene expression and loss of liver sexual dimorphism in the long-lived Ames dwarf mice. Biochem Biophys Res Commun 332 1086-1100. - PubMed
-
- Améen C, Lindén D, Larsson BM, Mode A, Holmäng A, and Oscarsson J (2004) Effects of gender and GH secretory pattern on sterol regulatory element-binding protein-1c and its target genes in rat liver. Am J Physiol Endocrinol Metab 287 E1039-E1048. - PubMed
-
- Anderson GD (2005a) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44 989-1008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

