Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model
- PMID: 19483194
- PMCID: PMC2722985
- DOI: 10.1093/hmg/ddp261
Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model
Abstract
Abnormal N-methyl-d-aspartate receptor (NMDAR) function has been implicated in the pathophysiology of schizophrenia. d-serine is an important NMDAR modulator, and to elucidate the role of the d-serine synthesis enzyme serine racemase (Srr) in schizophrenia, we identified and characterized mice with an ENU-induced mutation that results in a complete loss of Srr activity and dramatically reduced d-serine levels. Mutant mice displayed behaviors relevant to schizophrenia, including impairments in prepulse inhibition, sociability and spatial discrimination. Behavioral deficits were exacerbated by an NMDAR antagonist and ameliorated by d-serine or the atypical antipsychotic clozapine. Expression profiling revealed that the Srr mutation influenced several genes that have been linked to schizophrenia and cognitive ability. Transcript levels altered by the Srr mutation were also normalized by d-serine or clozapine treatment. Furthermore, analysis of SRR genetic variants in humans identified a robust association with schizophrenia. This study demonstrates that aberrant Srr function and diminished d-serine may contribute to schizophrenia pathogenesis.
Figures

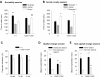
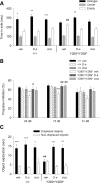
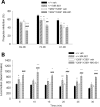
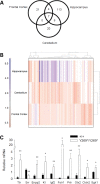
References
-
- Lewis D.A., Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nature Med. 2006;12:1016–1022. - PubMed
-
- Ross C.A., Margolis R.L., Reading S.A., Pletnikov M., Coyle J.T. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. - PubMed
-
- Javitt D.C., Zukin S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

