Radiotherapy combined with intratumoral dendritic cell vaccination enhances the therapeutic efficacy of adoptive T-cell transfer
- PMID: 19483649
- PMCID: PMC2743975
- DOI: 10.1097/CJI.0b013e3181a95165
Radiotherapy combined with intratumoral dendritic cell vaccination enhances the therapeutic efficacy of adoptive T-cell transfer
Abstract
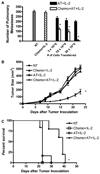
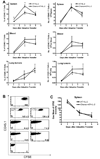
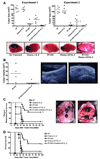
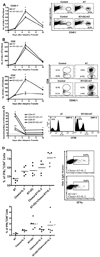
Treatment of C57BL/6 mice with cyclophosphamide (100 mg/kg) and fludarabine (200 mg/kg) induced nonmyeloablative lymphodepletion without inhibiting D5 melanoma tumor growth. Using this model, we found that induction of lymphopenia before adoptive transfer of ex vivo anti-CD3/CD28 activated and interleukin-2 expanded D5-G6 tumor draining lymph node cells enhanced the antitumor efficacy of the infused cells in both pulmonary metastases and subcutaneous D5 bearing mice. However, induction of lymphopenia did not promote intratumoral or extratumoral proliferation or accumulation of the infused cells. We have previously shown that radiotherapy enhances the therapeutic efficacy of intratumoral unpulsed dendritic cell vaccination in subcutaneous murine tumor models by augmenting the induction of antitumor cellular immune responses. Here, we confirmed this finding in a murine metastatic melanoma liver tumor model. Furthermore, local tumor irradiation combined with intratumoral dendritic cell administration significantly enhanced the therapeutic efficacy of tumor-reactive T cell adoptive transfer in this lymphodepleted liver tumor model. This was evident by reduced liver tumor size, decreased incidence of spontaneous intra-abdominal metastasis, and prolonged survival, resulting in 46% of mice cured. This enhanced antitumor activity was associated with a selective increase in proliferation, accumulation, and function of CD4+ rather than CD8+ infused cells. This multimodality regimen may have translational applications for the treatment of human cancers.
Conflict of interest statement
Figures


Similar articles
-
CD122+CD8+ Treg suppress vaccine-induced antitumor immune responses in lymphodepleted mice.Eur J Immunol. 2010 May;40(5):1375-85. doi: 10.1002/eji.200839210. Eur J Immunol. 2010. PMID: 20186876 Free PMC article.
-
Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma.J Immunother. 2008 May;31(4):402-12. doi: 10.1097/CJI.0b013e31816cabbb. J Immunother. 2008. PMID: 18391755
-
Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy.J Immunol. 2000 Oct 15;165(8):4246-53. doi: 10.4049/jimmunol.165.8.4246. J Immunol. 2000. PMID: 11035058
-
Dendritic cell recovery post-lymphodepletion: a potential mechanism for anti-cancer adoptive T cell therapy and vaccination.Cancer Immunol Immunother. 2010 Mar;59(3):341-53. doi: 10.1007/s00262-009-0792-6. Epub 2009 Nov 18. Cancer Immunol Immunother. 2010. PMID: 19921513 Free PMC article. Review.
-
Post-transplant adoptive T-cell immunotherapy.Best Pract Res Clin Haematol. 2008 Sep;21(3):503-19. doi: 10.1016/j.beha.2008.07.001. Best Pract Res Clin Haematol. 2008. PMID: 18790452 Free PMC article. Review.
Cited by
-
A Novel Therapeutic Tumor Vaccine Targeting MUC1 in Combination with PD-L1 Elicits Specific Anti-Tumor Immunity in Mice.Vaccines (Basel). 2022 Jul 8;10(7):1092. doi: 10.3390/vaccines10071092. Vaccines (Basel). 2022. PMID: 35891256 Free PMC article.
-
Clinical response of advanced cancer patients to cellular immunotherapy and intensity-modulated radiation therapy.Oncoimmunology. 2013 Oct 1;2(10):e26381. doi: 10.4161/onci.26381. Epub 2013 Oct 17. Oncoimmunology. 2013. PMID: 24349874 Free PMC article.
-
Cell transfer therapy for cancer: past, present, and future.J Immunol Res. 2014;2014:525913. doi: 10.1155/2014/525913. Epub 2014 Jan 9. J Immunol Res. 2014. PMID: 24741604 Free PMC article. Review.
-
Concurrent dendritic cell vaccine and strontium-89 radiation therapy in the management of multiple bone metastases.Ir J Med Sci. 2015 Jun;184(2):457-61. doi: 10.1007/s11845-014-1145-9. Epub 2014 May 30. Ir J Med Sci. 2015. PMID: 24876093
-
Combination Strategies to Optimize Efficacy of Dendritic Cell-Based Immunotherapy.Front Immunol. 2018 Dec 5;9:2759. doi: 10.3389/fimmu.2018.02759. eCollection 2018. Front Immunol. 2018. PMID: 30568653 Free PMC article. Review.
References
-
- Arciero CA, Sigurdson ER. Diagnosis and treatment of metastatic disease to the liver. Semin Oncol. 2008;35:147–159. - PubMed
-
- Young SE, Martinez SR, Essner R. The role of surgery in treatment of stage IV melanoma. J Surg Oncol. 2006;94:344–351. - PubMed
-
- Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. - PubMed
-
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. - PubMed
-
- Li Q, Chang AE. Adoptive T-cell immunotherapy of cancer. Cytokines Cell Mol Ther. 1999;5:105–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

