Odor quality coding and categorization in human posterior piriform cortex
- PMID: 19483688
- PMCID: PMC2834563
- DOI: 10.1038/nn.2324
Odor quality coding and categorization in human posterior piriform cortex
Abstract
Efficient recognition of odorous objects universally shapes animal behavior and is crucial for survival. To distinguish kin from nonkin, mate from nonmate and food from nonfood, organisms must be able to create meaningful perceptual representations of odor qualities and categories. It is currently unknown where and in what form the brain encodes information about odor quality. By combining functional magnetic resonance imaging (fMRI) with multivariate (pattern-based) techniques, we found that spatially distributed ensemble activity in human posterior piriform cortex (PPC) coincides with perceptual ratings of odor quality, such that odorants with more (or less) similar fMRI patterns were perceived as more (or less) alike. We did not observe these effects in anterior piriform cortex, amygdala or orbitofrontal cortex, indicating that ensemble coding of odor categorical perception is regionally specific for PPC. These findings substantiate theoretical models emphasizing the importance of distributed piriform templates for the perceptual reconstruction of odor object quality.
Figures
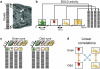

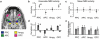
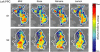
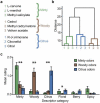


Comment in
-
A noseful of objects.Nat Neurosci. 2009 Jul;12(7):813-4. doi: 10.1038/nn0709-813. Nat Neurosci. 2009. PMID: 19554043 No abstract available.
References
-
- Rosch EH. Principles of categorization. In: Rosch EH, Lloyd B, editors. Cognition and Categorization. Erlbaum Associates; Hillsdale: 1978. pp. 27–48.
-
- Miller EK, Nieder A, Freedman DJ, Wallis JD. Neural correlates of categories and concepts. Curr. Opin. Neurobiol. 2003;13:198–203. - PubMed
-
- Reddy L, Kanwisher N. Coding of visual objects in the ventral stream. Curr. Opin. Neurobiol. 2006;16:408–414. - PubMed
-
- Kendrick KM, et al. Neural control of maternal behaviour and olfactory recognition of offspring. Brain Res. Bull. 1997;44:383–395. - PubMed
-
- Todrank J, Heth G, Johnston RE. Kin recognition in golden hamsters: evidence for kinship odours. Anim. Behav. 1998;55:377–386. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

