Pim-1 plays a pivotal role in hypoxia-induced chemoresistance
- PMID: 19483729
- PMCID: PMC3358117
- DOI: 10.1038/onc.2009.124
Pim-1 plays a pivotal role in hypoxia-induced chemoresistance
Abstract
Hypoxia changes the responses of cancer cells to many chemotherapy agents, resulting in chemoresistance. The underlying molecular mechanism of hypoxia-induced drug resistance remains unclear. Pim-1 is a survival kinase, which phosphorylates Bad at serine 112 to antagonize drug-induced apoptosis. Here we show that hypoxia increases Pim-1 in a hypoxia-inducible factor-1alpha-independent manner. Inhibition of Pim-1 function by dominant-negative Pim-1 dramatically restores the drug sensitivity to apoptosis induced by chemotherapy under hypoxic conditions in both in vitro and in vivo tumor models. Introduction of siRNAs for Pim-1 also resensitizes cancer cells to chemotherapy drugs under hypoxic conditions, whereas forced overexpression of Pim-1 endows solid tumor cells with resistance to cisplatin, even under normoxia. Dominant-negative Pim-1 prevents a decrease in mitochondrial transmembrane potential in solid tumor cells, which is normally induced by cisplatin (CDDP), followed by the reduced activity of Caspase-3 and Caspase-9, indicating that Pim-1 participates in hypoxia-induced drug resistance through the stabilization of mitochondrial transmembrane potential. Our results demonstrate that Pim-1 is a pivotal regulator involved in hypoxia-induced chemoresistance. Targeting Pim-1 may improve the chemotherapeutic strategy for solid tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

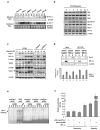
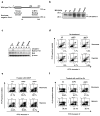
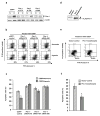

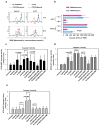
References
-
- Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. Pim-1 kinase promotes inactivation of the pro-apoptotic Bad protein by phosphorylating it on the Ser112 gatekeeper site. FEBS Lett. 2004;571:43–9. - PubMed
-
- Arsham AM, Plas DR, Thompson CB, Simon MC. Akt and hypoxia-inducible factor-1 independently enhance tumor growth and angiogenesis. Cancer Res. 2004;64:3500–7. - PubMed
-
- Bandyopadhyay RS, Phelan M, Faller DV. Hypoxia induces AP-1-regulated genes and AP-1 transcription factor binding in human endothelial and other cell types. Biochim Biophys Acta. 1995;1264:72–8. - PubMed
-
- Cardenas-Navia LI, Yu D, Braun RD, Brizel DM, Secomb TW, Dewhirst MW. Tumor-dependent kinetics of partial pressure of oxygen fluctuations during air and oxygen breathing. Cancer Res. 2004;64:6010–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

