Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors
- PMID: 19484140
- PMCID: PMC2685440
- DOI: 10.1593/neo.81578
Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors
Abstract
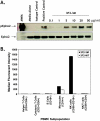
EphA2 is a receptor tyrosine kinase that has been shown to be overexpressed in a variety of human tumor types. Previous studies demonstrated that agonist monoclonal antibodies targeting EphA2 induced the internalization and degradation of the receptor, thereby abolishing its oncogenic effects. In this study, the in vitro and in vivo antibody-dependent cell-mediated cytotoxicity (ADCC) activity of EphA2 effector-enhanced agonist monoclonal antibodies was evaluated. With tumor cell lines and healthy human peripheral blood monocytes, the EphA2 antibodies demonstrated approximately 80% tumor cell killing. In a dose-dependent manner, natural killer (NK) cells were required for the in vitro ADCC activity and became activated as demonstrated by the induction of cell surface expression of CD107a. To assess the role of NK cells on antitumor efficacy in vivo, the EphA2 antibodies were evaluated in xenograft models in severe compromised immunodeficient (SCID) mice (which have functional NK cells and monocytes) and SCID nonobese diabetic (NOD) mice (which largely lack functional NK cells and monocytes). Dosing of EphA2 antibody in the SCID murine tumor model resulted in a 6.2-fold reduction in tumor volume, whereas the SCID/nonobese diabetic model showed a 1.6-fold reduction over the isotype controls. Together, these results demonstrate that the anti-EphA2 monoclonal antibodies may function through at least two mechanisms of action: EphA2 receptor activation and ADCC-mediated activity. These novel EphA2 monoclonal antibodies provide additional means by which host effector mechanisms can be activated for selective destruction of EphA2-expressing tumor cells.
Figures





Similar articles
-
Novel anti-EPHA2 antibody, DS-8895a for cancer treatment.Cancer Biol Ther. 2016 Nov;17(11):1158-1167. doi: 10.1080/15384047.2016.1235663. Epub 2016 Sep 21. Cancer Biol Ther. 2016. PMID: 27653549 Free PMC article.
-
Generation and preclinical characterization of an NKp80-Fc fusion protein for redirected cytolysis of natural killer (NK) cells against leukemia.J Biol Chem. 2015 Sep 11;290(37):22474-84. doi: 10.1074/jbc.M115.678912. Epub 2015 Jul 21. J Biol Chem. 2015. PMID: 26198633 Free PMC article.
-
Effects of interleukin-18 on natural killer cells: costimulation of activation through Fc receptors for immunoglobulin.Cancer Immunol Immunother. 2013 Jun;62(6):1073-82. doi: 10.1007/s00262-013-1403-0. Epub 2013 Apr 19. Cancer Immunol Immunother. 2013. PMID: 23604103 Free PMC article.
-
The "less-is-more" in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity.MAbs. 2018 Jul;10(5):693-711. doi: 10.1080/19420862.2018.1466767. MAbs. 2018. PMID: 29733746 Free PMC article. Review.
-
ADCC: the rock band led by therapeutic antibodies, tumor and immune cells.Front Immunol. 2025 Apr 16;16:1548292. doi: 10.3389/fimmu.2025.1548292. eCollection 2025. Front Immunol. 2025. PMID: 40308580 Free PMC article. Review.
Cited by
-
A comprehensive mathematical model for three-body binding equilibria.J Am Chem Soc. 2013 Apr 24;135(16):6092-9. doi: 10.1021/ja311795d. Epub 2013 Apr 16. J Am Chem Soc. 2013. PMID: 23544844 Free PMC article.
-
The antitumor activity of the human FOLR1-specific monoclonal antibody, farletuzumab, in an ovarian cancer mouse model is mediated by antibody-dependent cellular cytotoxicity.Cancer Biol Ther. 2013 Nov;14(11):1032-8. doi: 10.4161/cbt.26106. Epub 2013 Aug 28. Cancer Biol Ther. 2013. PMID: 24025360 Free PMC article.
-
Enhancement of antibody-dependent cell-mediated cytotoxicity by endowing IgG with FcαRI (CD89) binding.MAbs. 2015;7(4):743-51. doi: 10.1080/19420862.2015.1047570. MAbs. 2015. PMID: 25970007 Free PMC article.
-
Immunohistochemical expression of ephrin receptors in neuroendocrine neoplasms: a case-series of gastroenteropancreatic neuroendocrine neoplasms and a systematic review of the literature.Endocrine. 2025 Mar;87(3):1323-1332. doi: 10.1007/s12020-024-04079-6. Epub 2024 Oct 19. Endocrine. 2025. PMID: 39425842
-
The interconnectedness of cancer cell signaling.Neoplasia. 2011 Dec;13(12):1183-93. doi: 10.1593/neo.111746. Neoplasia. 2011. PMID: 22241964 Free PMC article.
References
-
- Zhang J, Hughes S. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol. 2006;208:453–461. - PubMed
-
- Heroult M, Schaffner F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. - PubMed
-
- Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–157. - PubMed
-
- Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J, Mohammed SI. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res. 2006;12:353–360. - PubMed
-
- Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–551. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
