Urokinase-type plasminogen activator receptor transcriptionally controlled adenoviruses eradicate pancreatic tumors and liver metastasis in mouse models
- PMID: 19484141
- PMCID: PMC2685441
- DOI: 10.1593/neo.81674
Urokinase-type plasminogen activator receptor transcriptionally controlled adenoviruses eradicate pancreatic tumors and liver metastasis in mouse models
Abstract
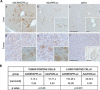
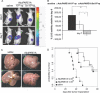
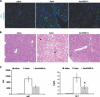
Treatment options for pancreatic cancer have shown limited success mainly owing to poor selectivity for pancreatic tumor tissue and to a lack of activity in the tumor. In this study, we describe the ability of the urokinase-type plasminogen activator receptor (uPAR) promoter to efficiently and selectively target pancreatic tumors and metastases, which enables the successful management of pancreatic cancer. We have generated a replication-defective reporter adenovirus, AduPARLuc, and a conditionally replicating adenovirus, AduPARE1A, and we have studied the selectivity and antitumoral efficacy in pancreatic tumors and metastases. Toxicity was studied on intravascular delivery. We demonstrate that the uPAR promoter is highly active in pancreatic tumors but very weak in normal tissues. Tumor specificity is evidenced by a 100-fold increase in the tumor-to-liver ratio and by selective targeting of liver metastases (P < .001). Importantly, the AduPARE1A maintains the oncolytic activity of the wild-type virus, with reduced toxicity, and exhibits significant antitumoral activity (25% tumor eradication) and prolonged survival in pancreatic xenograft models (P < .0001). Furthermore, upon intravascular delivery, we demonstrate complete eradication of liver metastasis in 33% of mice, improving median survival (P = 5.43 x 10(-5)). The antitumoral selective activity of AduPARE1A shows the potential of uPAR promoter-based therapies in pancreatic cancer treatment.
Figures



References
-
- Rustgi AK. The molecular pathogenesis of pancreatic cancer: clarifying a complex circuitry. Genes Dev. 2006;20:3043–3053. - PubMed
-
- Saif MW. Pancreatic cancer: are we moving forward yet? Highlights from the Gastrointestinal Cancers Symposium. Orlando, FL, USA. January 20th, 2007. JOP. 2007;8:166–176. - PubMed
-
- Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. - PubMed
-
- Nemunaitis J, Senzer N, Sarmiento S, Zhang YA, Arzaga R, Sands B, Maples P, Tong AW. A phase I trial of intravenous infusion of ONYX-015 and enbrel in solid tumor patients. Cancer Gene Ther. 2007;14:885–893. - PubMed
-
- Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
