Mammalian iron transport
- PMID: 19484405
- PMCID: PMC11115736
- DOI: 10.1007/s00018-009-0051-1
Mammalian iron transport
Abstract
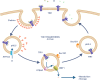
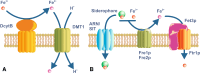
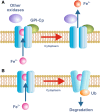
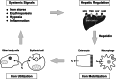
Iron is essential for basic cellular processes but is toxic when present in excess. Consequently, iron transport into and out of cells is tightly regulated. Most iron is delivered to cells bound to plasma transferrin via a process that involves transferrin receptor 1, divalent metal-ion transporter 1 and several other proteins. Non-transferrin-bound iron can also be taken up efficiently by cells, although the mechanism is poorly understood. Cells can divest themselves of iron via the iron export protein ferroportin in conjunction with an iron oxidase. The linking of an oxidoreductase to a membrane permease is a common theme in membrane iron transport. At the systemic level, iron transport is regulated by the liver-derived peptide hepcidin which acts on ferroportin to control iron release to the plasma.
Figures
References
-
- Crosa JH, Mey AR, Payne SM, editors. Iron transport in bacteria. Washington, DC: American Society for Microbiology; 2004.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

