Carboplatin-based primary chemotherapy for infants and young children with CNS tumors
- PMID: 19484793
- PMCID: PMC4307774
- DOI: 10.1002/cncr.24362
Carboplatin-based primary chemotherapy for infants and young children with CNS tumors
Abstract
Background: A carboplatin-based chemotherapy regimen was used as primary postoperative therapy in infants with central nervous system (CNS) tumors to limit renal and ototoxicity and to target systemic exposure.
Methods: Fifty-three patients aged <age 3 years with embryonal CNS tumor medulloblastoma (n = 20), ependymoma (EP, n = 21), choroid plexus carcinoma (CPCA, n = 5), and primitive embryonal neoplasms including atypical teratoid rhabdoid tumors (n = 7) were treated with cyclophosphamide, etoposide, and carboplatin. Radiation therapy was used only for residual disease at the end of chemotherapy or disease progression.
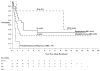
Results: The response rate after 2 cycles of chemotherapy was 34% (complete response, 13.8%; partial response, 20.7%). Myelosuppression was the dominant toxicity; 2 patients had toxic deaths related to thrombocytopenia with trauma. The 5-year overall survival (OS) was 49% +/- 7%, and the progression-free survival (PFS) was 31% +/- 7%, with a median follow-up of 11.4 years (range, 5.2-15.0 years). For medulloblastoma, the 5-year PFS was 26% +/- 9%; for EP it was 33% +/- 10%; for CPCA it was 80% +/- 18%; and for primitive neuroectodermal and atypical teratoid rhabdoid tumors it was 0%. Localized EP patients with gross total resection who did not undergo radiotherapy had a 5-year PFS of 57% +/- 17% and OS of 71% +/- 16%. Two patients developed late second malignancies; 1 was associated with germline p53 mutation.
Conclusions: The results confirm that carboplatin has similar activity to cisplatin in otherwise similar regimens. Five-year survival data are comparable to those reported in other recent studies, including high-dose chemotherapy studies. Of note is the marked activity in CPCA and gross totally resected EP.
Figures
References
-
- Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–31. - PubMed
-
- Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23:7621–31. - PubMed
-
- Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–86. - PubMed
-
- Grill J, Sainte-Rose C, Jouvet A, Gentet JC, Lejars O, Frappaz D, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6:573–80. - PubMed
-
- Grill J, Le Deley MC, Gambarelli D, Raquin MA, Couanet D, Pierre-Kahn A, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19:1288–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous