Functional roles of the pre-sensor I insertion sequence in an AAA+ bacterial enhancer binding protein
- PMID: 19486295
- PMCID: PMC2745333
- DOI: 10.1111/j.1365-2958.2009.06744.x
Functional roles of the pre-sensor I insertion sequence in an AAA+ bacterial enhancer binding protein
Abstract
Molecular machines belonging to the AAA+ superfamily of ATPases use NTP hydrolysis to remodel their versatile substrates. The presence of an insertion sequence defines the major phylogenetic pre-sensor I insertion (pre-SIi) AAA+ superclade. In the bacterial sigma(54)-dependent enhancer binding protein phage shock protein F (PspF) the pre-SIi loop adopts different conformations depending on the nucleotide-bound state. Single amino acid substitutions within the dynamic pre-SIi loop of PspF drastically change the ATP hydrolysis parameters, indicating a structural link to the distant hydrolysis site. We used a site-specific protein-DNA proximity assay to measure the contribution of the pre-SIi loop in sigma(54)-dependent transcription and demonstrate that the pre-SIi loop is a major structural feature mediating nucleotide state-dependent differential engagement with Esigma(54). We suggest that much, if not all, of the action of the pre-SIi loop is mediated through the L1 loop and relies on a conserved molecular switch, identified in a crystal structure of one pre-SIi variant and in accordance with the high covariance between some pre-SIi residues and distinct residues outside the pre-SIi sequence.
Figures


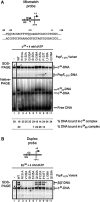

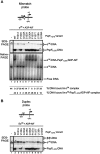
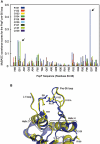
References
-
- Burrows PC, Wigneshweraraj SR, Buck M. Protein–DNA interactions that govern AAA+ activator-dependent bacterial transcription initiation. J Mol Biol. 2008;375:43–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

