Cognate Site Identifier analysis reveals novel binding properties of the Sex Inducer homeodomain proteins of Cryptococcus neoformans
- PMID: 19486297
- PMCID: PMC2776684
- DOI: 10.1111/j.1365-2958.2009.06719.x
Cognate Site Identifier analysis reveals novel binding properties of the Sex Inducer homeodomain proteins of Cryptococcus neoformans
Abstract
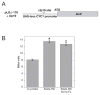
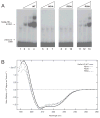
Homeodomain proteins function in fungi to specify cell types and control sexual development. In the meningoencephalitis-causing fungal pathogen Cryptococcus neoformans, sexual development leads to the production of spores (suspected infectious particles). Sexual development is controlled by the homeodomain transcription factors Sxi1alpha and Sxi2a, but the mechanism by which they act is unknown. To understand how the Sxi proteins regulate development, we characterized their binding properties in vitro, showing that Sxi2a does not require a partner to bind DNA with high affinity. We then utilized a novel approach, Cognate Site Identifier (CSI) arrays, to define a comprehensive DNA-binding profile for Sxi2a, revealing a consensus sequence distinct from those of other fungal homeodomain proteins. Finally, we show that the homeodomains of both Sxi proteins are required for sexual development, a departure from related fungi. Our findings support a model in which Sxi1alpha and Sxi2a control sexual development in a homeodomain-dependent manner by binding to DNA sequences that differ from those defined in previously established fungal paradigms.
Figures



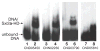

References
-
- Alspaugh JA, Perfect JR, Heitman J. Signal transduction pathways regulating differentiation and pathogenicity of Cryptococcus neoformans. Fungal Genet Biol. 1998;25:1–14. - PubMed
-
- Asante-Owusu RN, Banham AH, Bohnert HU, Mellor EJ, Casselton LA. Heterodimerization between two classes of homeodomain proteins in the mushroom Coprinus cinereus brings together potential DNA-binding and activation domains. Gene. 1996;172:25–31. - PubMed
-
- Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current Protocols in Molecular Biology. Boston, MA: John Wiley and Sons; 1997.
-
- Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol. 1994;2:28–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

