Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice
- PMID: 19486528
- PMCID: PMC2694181
- DOI: 10.1186/1465-9921-10-43
Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice
Abstract
Background: The immune mechanisms associated with infection-induced disease exacerbations in asthma and COPD are not fully understood. Toll-like receptor (TLR) 3 has an important role in recognition of double-stranded viral RNA, which leads to the production of various inflammatory mediators. Thus, an understanding of TLR3 activation should provide insight into the mechanisms underlying virus-induced exacerbations of pulmonary diseases.
Methods: TLR3 knock-out (KO) mice and C57B6 (WT) mice were intranasally administered repeated doses of the synthetic double stranded RNA analog poly(I:C).
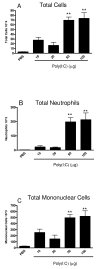
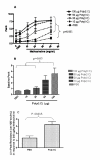
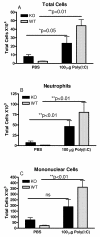
Results: There was a significant increase in total cells, especially neutrophils, in BALF samples from poly(I:C)-treated mice. In addition, IL-6, CXCL10, JE, KC, mGCSF, CCL3, CCL5, and TNFalpha were up regulated. Histological analyses of the lungs revealed a cellular infiltrate in the interstitium and epithelial cell hypertrophy in small bronchioles. Associated with the pro-inflammatory effects of poly(I:C), the mice exhibited significant impairment of lung function both at baseline and in response to methacholine challenge as measured by whole body plethysmography and an invasive measure of airway resistance. Importantly, TLR3 KO mice were protected from poly(I:C)-induced changes in lung function at baseline, which correlated with milder inflammation in the lung, and significantly reduced epithelial cell hypertrophy.
Conclusion: These findings demonstrate that TLR3 activation by poly(I:C) modulates the local inflammatory response in the lung and suggest a critical role of TLR3 activation in driving lung function impairment. Thus, TLR3 activation may be one mechanism through which viral infections contribute toward exacerbation of respiratory disease.
Figures

Similar articles
-
Toll-like receptor 3 stimulation causes corticosteroid-refractory airway neutrophilia and hyperresponsiveness in mice.Chest. 2013 Jul;144(1):99-105. doi: 10.1378/chest.12-2610. Chest. 2013. PMID: 23348232
-
Effect of TLR3/dsRNA complex inhibitor on Poly(I:C)-induced airway inflammation in Swiss albino mice.Environ Sci Pollut Res Int. 2023 Feb;30(10):28118-28132. doi: 10.1007/s11356-022-23987-6. Epub 2022 Nov 17. Environ Sci Pollut Res Int. 2023. PMID: 36394807
-
Oxidative Stress Attenuates TLR3 Responsiveness and Impairs Anti-viral Mechanisms in Bronchial Epithelial Cells From COPD and Asthma Patients.Front Immunol. 2019 Nov 29;10:2765. doi: 10.3389/fimmu.2019.02765. eCollection 2019. Front Immunol. 2019. PMID: 31849956 Free PMC article.
-
TLR3: interferon induction by double-stranded RNA including poly(I:C).Adv Drug Deliv Rev. 2008 Apr 29;60(7):805-12. doi: 10.1016/j.addr.2007.11.005. Epub 2008 Jan 2. Adv Drug Deliv Rev. 2008. PMID: 18262679 Review.
-
Toll-Like Receptor 3 in Cardiovascular Diseases.Heart Lung Circ. 2022 Jul;31(7):e93-e109. doi: 10.1016/j.hlc.2022.02.012. Epub 2022 Mar 30. Heart Lung Circ. 2022. PMID: 35367134 Review.
Cited by
-
Intranasal administration of poly(I:C) and LPS in BALB/c mice induces airway hyperresponsiveness and inflammation via different pathways.PLoS One. 2012;7(2):e32110. doi: 10.1371/journal.pone.0032110. Epub 2012 Feb 15. PLoS One. 2012. PMID: 22355412 Free PMC article.
-
Double-stranded RNA induces molecular and inflammatory signatures that are directly relevant to COPD.Mucosal Immunol. 2013 May;6(3):474-84. doi: 10.1038/mi.2012.86. Epub 2012 Sep 19. Mucosal Immunol. 2013. PMID: 22990623 Free PMC article.
-
Differential regulation of inflammation and immunity in mild and severe experimental asthma.Mediators Inflamm. 2013;2013:808470. doi: 10.1155/2013/808470. Epub 2013 May 27. Mediators Inflamm. 2013. PMID: 23781124 Free PMC article.
-
Particulate Matter Exposure Aggravates IL-17-Induced Eye and Nose Inflammation in an OVA/Poly(I:C) Mouse Model.Allergy Asthma Immunol Res. 2022 Jan;14(1):59-72. doi: 10.4168/aair.2022.14.1.59. Allergy Asthma Immunol Res. 2022. PMID: 34983107 Free PMC article.
-
TRIF Deficiency does not Affect Severity of Ovalbumin-induced Airway Inflammation in Mice.Immune Netw. 2014 Oct;14(5):249-54. doi: 10.4110/in.2014.14.5.249. Epub 2014 Oct 22. Immune Netw. 2014. PMID: 25360076 Free PMC article.
References
-
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101(10):3516–3521. doi: 10.1073/pnas.0400525101. Epub 2004 Mar 3511. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

