Two-electron reactions S2QB -->S0QB and S3QB -->S1QB are involved in deactivation of higher S states of the oxygen-evolving complex of Photosystem II
- PMID: 19486689
- PMCID: PMC2711488
- DOI: 10.1016/j.bpj.2009.03.007
Two-electron reactions S2QB -->S0QB and S3QB -->S1QB are involved in deactivation of higher S states of the oxygen-evolving complex of Photosystem II
Abstract
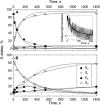
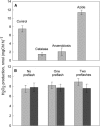
The oxygen-evolving complex of Photosystem II cycles through five oxidation states (S(0)-S(4)), and dark incubation leads to 25% S(0) and 75% S(1). This distribution cannot be reached with charge recombination reactions between the higher S states and the electron acceptor Q(B)(-). We measured flash-induced oxygen evolution to understand how S(3) and S(2) are converted to lower S states when the electron required to reduce the manganese cluster does not come from Q(B)(-). Thylakoid samples preconditioned to make the concentration of the S(1) state 100% and to oxidize tyrosine Y(D) were illuminated by one or two laser preflashes, and flash-induced oxygen evolution sequences were recorded at various time intervals after the preflashes. The distribution of the S states was calculated from the flash-induced oxygen evolution pattern using an extended Kok model. The results suggest that S(2) and S(3) are converted to lower S states via recombination from S(2)Q(B)(-) and S(3)Q(B)(-) and by a slow change of the state of oxygen-evolving complex from S(3) and S(2) to S(1) and S(0) in reactions with unspecified electron donors. The slow pathway appears to contain two-electron routes, S(2)Q(B) -->S(0)Q(B), and S(3)Q(B) -->S(1)Q(B). The two-electron reactions dominate in intact thylakoid preparations in the absence of chemical additives. The two-electron reaction was replaced by a one-electron-per-step pathway, S(3)Q(B) -->S(2)Q(B) -->S(1)Q(B) in PS II-enriched membrane fragments and in thylakoids measured in the presence of artificial electron acceptors. A catalase effect suggested that H(2)O(2) acts as an electron donor for the reaction S(2)Q(B) -->S(0)Q(B) but added H(2)O(2) did not enhance this reaction.
Figures



References
-
- Kern J., Renger G. Photosystem II: Structure and mechanism of the water: plastoquinone oxidoreductase. Photosynth. Res. 2007;94:183–202. - PubMed
-
- Ferreira K.N., Iverson T.M., Maghlaoui K., Barber J., Iwata S. Architecture of the photosynthetic oxygen-evolving center. Science. 2004;303:1831–1838. - PubMed
-
- Barber J. Crystal structure of the oxygen-evolving complex of photosystem II. Inorg. Chem. 2008;47:1700–1710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

