Connexin40 and connexin43 determine gating properties of atrial gap junction channels
- PMID: 19486903
- PMCID: PMC2813328
- DOI: 10.1016/j.yjmcc.2009.05.014
Connexin40 and connexin43 determine gating properties of atrial gap junction channels
Abstract
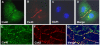
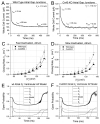
While ventricular gap junctions contain only Cx43, atrial gap junctions contain both Cx40 and Cx43; yet the functional consequences of this co-expression remain poorly understood. We quantitated the expression of Cx40 and Cx43 and their contributions to atrial gap junctional conductance (g(j)). Neonatal murine atrial myocytes showed similar abundances of Cx40 and Cx43 proteins, while ventricular myocytes contained at least 20 times more Cx43 than Cx40. Since Cx40 gap junction channels are blocked by 2 mM spermine while Cx43 channels are unaffected, we used spermine block as a functional dual whole cell patch clamp assay to determine Cx40 contributions to cardiac g(j). Slightly more than half of atrial g(j) and <or=20% of ventricular g(j) were inhibited. In myocytes from Cx40 null mice, the inhibition of ventricular g(j) was completely abolished, and the block of atrial g(j) was reduced to <20%. Compared to ventricular gap junctions, the transjunctional voltage (V(j))-dependent inactivation of atrial g(j) was reduced and kinetically slowed, while the V(j)-dependence of fast and slow inactivation was unchanged. We conclude that Cx40 and Cx43 are equally abundant in atrium and make similar contributions to atrial g(j). Co-expression of Cx40 accounts for most, but not all, of the differences in the V(j)-dependent gating properties between atrium and ventricle that may play a role in the genesis of slow myocardial conduction and arrhythmias.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures



References
-
- Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2003;84:431–488. - PubMed
-
- Davis LM, Kanter HL, Beyer EC, Saffitz JE. Distinct gap junction phenotypes in cardiac tissues with disparate conduction properties. J Am Coll Cardiol. 1994;24:1124–1132. - PubMed
-
- Gros D, Jarry-Guichard T, Ten Velde I, de Maziere A, van Kempen MJ, Davoust J, Briand JP, Moorman AF, Jongsma HJ. Restricted distribution of connexin40, a gap junctional protein, in mammalian heart. Circ Res. 1994;74:839–851. 1994. - PubMed
-
- Firouzi M, Ramanna H, Kok B, Jongsma HJ, Koeleman BP, Doevendans PA, Groenewegen WA, Hauer RN. Association of human connexin40 gene polymorphisms with atrial vulnerability as a risk factor for idiopathic atrial fibrillation. Circ Res. 2004;95:e29–e33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

