Adaptive therapy
- PMID: 19487300
- PMCID: PMC3728826
- DOI: 10.1158/0008-5472.CAN-08-3658
Adaptive therapy
Abstract
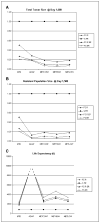
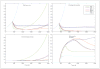
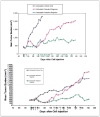
A number of successful systemic therapies are available for treatment of disseminated cancers. However, tumor response is often transient, and therapy frequently fails due to emergence of resistant populations. The latter reflects the temporal and spatial heterogeneity of the tumor microenvironment as well as the evolutionary capacity of cancer phenotypes to adapt to therapeutic perturbations. Although cancers are highly dynamic systems, cancer therapy is typically administered according to a fixed, linear protocol. Here we examine an adaptive therapeutic approach that evolves in response to the temporal and spatial variability of tumor microenvironment and cellular phenotype as well as therapy-induced perturbations. Initial mathematical models find that when resistant phenotypes arise in the untreated tumor, they are typically present in small numbers because they are less fit than the sensitive population. This reflects the "cost" of phenotypic resistance such as additional substrate and energy used to up-regulate xenobiotic metabolism, and therefore not available for proliferation, or the growth inhibitory nature of environments (i.e., ischemia or hypoxia) that confer resistance on phenotypically sensitive cells. Thus, in the Darwinian environment of a cancer, the fitter chemosensitive cells will ordinarily proliferate at the expense of the less fit chemoresistant cells. The models show that, if resistant populations are present before administration of therapy, treatments designed to kill maximum numbers of cancer cells remove this inhibitory effect and actually promote more rapid growth of the resistant populations. We present an alternative approach in which treatment is continuously modulated to achieve a fixed tumor population. The goal of adaptive therapy is to enforce a stable tumor burden by permitting a significant population of chemosensitive cells to survive so that they, in turn, suppress proliferation of the less fit but chemoresistant subpopulations. Computer simulations show that this strategy can result in prolonged survival that is substantially greater than that of high dose density or metronomic therapies. The feasibility of adaptive therapy is supported by in vivo experiments. [Cancer Res 2009;69(11):4894-903] Major FindingsWe present mathematical analysis of the evolutionary dynamics of tumor populations with and without therapy. Analytic solutions and numerical simulations show that, with pretreatment, therapy-resistant cancer subpopulations are present due to phenotypic or microenvironmental factors; maximum dose density chemotherapy hastens rapid expansion of resistant populations. The models predict that host survival can be maximized if "treatment-for-cure strategy" is replaced by "treatment-for-stability." Specifically, the models predict that an optimal treatment strategy will modulate therapy to maintain a stable population of chemosensitive cells that can, in turn, suppress the growth of resistant populations under normal tumor conditions (i.e., when therapy-induced toxicity is absent). In vivo experiments using OVCAR xenografts treated with carboplatin show that adaptive therapy is feasible and, in this system, can produce long-term survival.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


References
-
- Frei E, Canellos GP. Dose, a critical factor in cancer chemotherapy. Am J Med. 1980;69:585–95. - PubMed
-
- Frei E, Elias A, Wheeler C, et al. The relationship between high-dose treatment and combination chemotherapy. The concept of summation dose intensity. Clin Cancer Res. 1998;314:1423–31. - PubMed
-
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–92. - PubMed
-
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. - PubMed
-
- Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

