Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer
- PMID: 19487376
- PMCID: PMC2736070
- DOI: 10.1200/JCO.2009.19.6410
Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer
Abstract
Purpose: Population studies have suggested that metformin use in diabetic patients decreases cancer incidence and mortality. Metformin inhibits the growth of cancer cells in vitro and tumors in vivo. However, there is little clinical data to support this. Our purpose was to determine whether metformin use was associated with a change in pathologic complete response (pCR) rates in diabetic patients with breast cancer receiving neoadjuvant chemotherapy.
Patients and methods: We identified 2,529 patients who received neoadjuvant chemotherapy for early-stage breast cancer between 1990 and 2007. Patients were compared by groups: 68 diabetic patients taking metformin, 87 diabetic patients not taking metformin, and 2,374 nondiabetic patients. pCR rates were compared between the three groups using chi(2) tests of independence and compared pair- wise using a binomial test of proportions. Factors predictive of pCR were assessed using a multivariate logistic regression model.
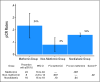
Results: The rate of pCR was 24% in the metformin group, 8.0% in the nonmetformin group, and 16% in the nondiabetic group (P = .02). Pairwise comparisons between the metformin and nonmetformin groups (P = .007) and the nonmetformin and nondiabetic groups (P = .04) were significant. Comparison of the pCR rates between the metformin and nondiabetic groups trended toward but did not meet significance (P = .10). Metformin use was independently predictive of pCR (odds ratio, 2.95; P = .04) after adjustment for diabetes, body mass index, age, stage, grade, receptor status, and neoadjuvant taxane use.
Conclusion: Diabetic patients with breast cancer receiving metformin and neoadjuvant chemotherapy have a higher pCR rate than do diabetics not receiving metformin. Additional studies to evaluate the potential of metformin as an antitumor agent are warranted.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Metformin in breast cancer: time for action.J Clin Oncol. 2009 Jul 10;27(20):3271-3. doi: 10.1200/JCO.2009.22.1630. Epub 2009 Jun 1. J Clin Oncol. 2009. PMID: 19487373 No abstract available.
-
Metformin as an addition to conventional chemotherapy in breast cancer.J Clin Oncol. 2009 Dec 10;27(35):e259; author reply e260. doi: 10.1200/JCO.2009.25.4110. Epub 2009 Nov 2. J Clin Oncol. 2009. PMID: 19884518 No abstract available.
-
Metformin, B(12), and enhanced breast cancer response to chemotherapy.J Clin Oncol. 2010 Jan 10;28(2):e19; author reply e20. doi: 10.1200/JCO.2009.25.7857. Epub 2009 Nov 30. J Clin Oncol. 2010. PMID: 19949002 No abstract available.
References
-
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. - PubMed
-
- Wolf I, Sadetzki S, Catane R, et al. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6:103–111. - PubMed
-
- Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: A review of the current evidence. Am J Clin Nutr. 2007;86:s823–s835. - PubMed
-
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int J Cancer. 2007;121:856–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical