Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial
- PMID: 19487385
- PMCID: PMC4979080
- DOI: 10.1200/JCO.2007.14.3339
Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial
Abstract
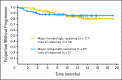
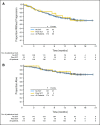
PURPOSE Patients with chronic myelogenous leukemia in accelerated phase (CML-AP) that is resistant or intolerant to imatinib have limited therapeutic options. Dasatinib, a potent inhibitor of BCR-ABL and SRC-family kinases, has efficacy in patients with CML-AP who have experienced treatment failure with imatinib. We now report follow-up data from the full patient cohort of 174 patients enrolled onto a phase II trial to provide a more complete assessment of the efficacy and safety of dasatinib in this population. PATIENTS AND METHODS Patients with imatinib-resistant (n = 161) or -intolerant (n = 13) CML-AP received dasatinib 70 mg orally twice daily. Results At a median follow-up of 14.1 months (treatment duration, 0.1 to 21.7 months), major and complete hematologic responses were attained by 64% and 45% of patients, respectively, and major and complete cytogenetic responses were achieved in 39% and 32% of patients, respectively. Responses were achieved irrespective of imatinib status (resistant or intolerant), prior stem-cell transplantation, or the presence of prior BCR-ABL mutation. The 12-month progression-free survival and overall survival rates were 66% and 82%, respectively. Dasatinib was generally well tolerated; the most frequent nonhematologic severe treatment-related adverse event was diarrhea (52%; grade 3 to 4, 8%). Cytopenias were common, including grade 3 to 4 neutropenia (76%) and thrombocytopenia (82%). Pleural effusion occurred in 27% of patients (grade 3 to 4, 5%). CONCLUSION Dasatinib is effective in patients with CML-AP after imatinib treatment failure.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2004. Bethesda, MD: National Cancer Institute; 2006. http://seer.cancer.gov/csr/1975_2004.
-
- Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. - PubMed
-
- Lahaye T, Riehm B, Berger U, et al. Response and resistance in 300 patients with BCR-ABL-positive leukemias treated with imatinib in a single center: A 4.5-year follow-up. Cancer. 2005;103:1659–1669. - PubMed
-
- Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: Results of a phase 2 study. Blood. 2002;99:1928–1937. - PubMed
-
- Kantarjian H, Talpaz M, O'Brien S, et al. Survival benefit with imatinib mesylate therapy in patients with accelerated-phase chronic myelogenous leukemia: Comparison with historic experience. Cancer. 2005;103:2099–2108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

