Priming of protective T cell responses against virus-induced tumors in mice with human immune system components
- PMID: 19487422
- PMCID: PMC2715061
- DOI: 10.1084/jem.20081720
Priming of protective T cell responses against virus-induced tumors in mice with human immune system components
Abstract
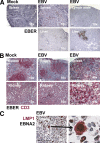
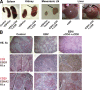
Many pathogens that cause human disease infect only humans. To identify the mechanisms of immune protection against these pathogens and also to evaluate promising vaccine candidates, a small animal model would be desirable. We demonstrate that primary T cell responses in mice with reconstituted human immune system components control infection with the oncogenic and persistent Epstein-Barr virus (EBV). These cytotoxic and interferon-gamma-producing T cell responses were human leukocyte antigen (HLA) restricted and specific for EBV-derived peptides. In HLA-A2 transgenic animals and similar to human EBV carriers, T cell responses against lytic EBV antigens dominated over recognition of latent EBV antigens. T cell depletion resulted in elevated viral loads and emergence of EBV-associated lymphoproliferative disease. Both loss of CD4(+) and CD8(+) T cells abolished immune control. Therefore, this mouse model recapitulates features of symptomatic primary EBV infection and generates T cell-mediated immune control that resists oncogenic transformation.
Figures





References
-
- Mestas J., Hughes C.C. 2004. Of mice and not men: differences between mouse and human immunology.J. Immunol. 172:2731–2738 - PubMed
-
- Waterston R.H., Lindblad-Toh K., Birney E., Rogers J., Abril J.F., Agarwal P., Agarwala R., Ainscough R., Alexandersson M., An P., et al. 2002. Initial sequencing and comparative analysis of the mouse genome.Nature. 420:520–562 - PubMed
-
- Young L.S., Rickinson A.B. 2004. Epstein-Barr virus: 40 years on.Nat. Rev. Cancer. 4:757–768 - PubMed
-
- Cohen J.I. 2000. Epstein-Barr virus infection.N. Engl. J. Med. 343:481–492 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

