Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism
- PMID: 19487623
- PMCID: PMC4112556
- DOI: 10.1001/archgenpsychiatry.2009.30
Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism
Abstract
Context: Selective serotonin reuptake inhibitors are widely prescribed for children with autism spectrum disorders.
Objectives: To determine the efficacy and safety of citalopram hydrobromide therapy for repetitive behavior in children with autism spectrum disorders.
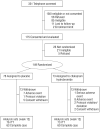
Design: National Institutes of Health-sponsored randomized controlled trial.
Setting: Six academic centers, including Mount Sinai School of Medicine, North Shore-Long Island Jewish Health System, University of North Carolina at Chapel Hill, University of California at Los Angeles, Yale University, and Dartmouth Medical School.
Participants: One hundred forty-nine volunteers 5 to 17 years old (mean [SD] age, 9.4 [3.1] years) were randomized to receive citalopram (n = 73) or placebo (n = 76). Participants had autistic spectrum disorders, Asperger disorder, or pervasive developmental disorder, not otherwise specified; had illness severity ratings of at least moderate on the Clinical Global Impressions, Severity of Illness Scale; and scored at least moderate on compulsive behaviors measured with the Children's Yale-Brown Obsessive Compulsive Scales modified for pervasive developmental disorders.
Interventions: Twelve weeks of citalopram hydrobromide (10 mg/5 mL) or placebo. The mean (SD) maximum dosage of citalopram hydrobromide was 16.5 (6.5) mg/d by mouth (maximum, 20 mg/d).
Main outcome measures: Positive response was defined by a score of much improved or very much improved on the Clinical Global Impressions, Improvement subscale. An important secondary outcome was the score on the Children's Yale-Brown Obsessive Compulsive Scales modified for pervasive developmental disorders. Adverse events were systematically elicited using the Safety Monitoring Uniform Report Form.
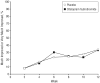
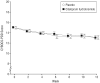
Results: There was no significant difference in the rate of positive response on the Clinical Global Impressions, Improvement subscale between the citalopram-treated group (32.9%) and the placebo group (34.2%) (relative risk, 0.96; 95% confidence interval, 0.61-1.51; P > .99). There was no difference in score reduction on the Children's Yale-Brown Obsessive Compulsive Scales modified for pervasive developmental disorders from baseline (mean [SD], -2.0 [3.4] points for the citalopram-treated group and -1.9 [2.5] points for the placebo group; P = .81). Citalopram use was significantly more likely to be associated with adverse events, particularly increased energy level, impulsiveness, decreased concentration, hyperactivity, stereotypy, diarrhea, insomnia, and dry skin or pruritus.
Conclusion: Results of this trial do not support the use of citalopram for the treatment of repetitive behavior in children and adolescents with autism spectrum disorders. Trial Registration clinicaltrials.gov Identifier: NCT00086645.
Figures
Comment in
-
Citalopram treatment in children with autism spectrum disorders and high levels of repetitive behavior.Arch Gen Psychiatry. 2009 Jun;66(6):581-2. doi: 10.1001/archgenpsychiatry.2009.42. Arch Gen Psychiatry. 2009. PMID: 19487622 No abstract available.
-
Citalopram not effective for repetitive behaviour in autistic spectrum disorders.Evid Based Ment Health. 2010 Feb;13(1):22. doi: 10.1136/ebmh.13.1.22. Evid Based Ment Health. 2010. PMID: 20164519 No abstract available.
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4th. Washington, DC: American Psychiatric Association; 2000.
-
- Johnson CP, Myers SM American Academy of Pediatrics Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120(5):1183–1215. - PubMed
-
- Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2007;17(3):348–355. - PubMed
-
- King BH, Bostic JQ. An update on pharmacologic treatments for autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2006;15(1):161–175. - PubMed
-
- Richler J, Bishop SL, Kleinke JR, Lord C. Restricted and repetitive behaviors in young children with autism spectrum disorders. J Autism Dev Disord. 2007;37(1):73–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

