Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate
- PMID: 19487664
- PMCID: PMC2705607
- DOI: 10.1073/pnas.0904113106
Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate
Abstract
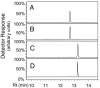
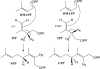
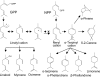
We identified a cis-prenyltransferase gene, neryl diphosphate synthase 1 (NDPS1), that is expressed in cultivated tomato (Solanum lycopersicum) cultivar M82 type VI glandular trichomes and encodes an enzyme that catalyzes the formation of neryl diphosphate from isopentenyl diphosphate and dimethylallyl diphosphate. mRNA for a terpene synthase gene, phellandrene synthase 1 (PHS1), was also identified in these glands. It encodes an enzyme that uses neryl diphosphate to produce beta-phellandrene as the major product as well as a variety of other monoterpenes. The profile of monoterpenes produced by PHS1 is identical with the monoterpenes found in type VI glands. PHS1 and NDPS1 map to chromosome 8, and the presence of a segment of chromosome 8 derived from Solanum pennellii LA0716 causes conversion from the M82 gland monoterpene pattern to that characteristic of LA0716 plants. The data indicate that, contrary to the textbook view of geranyl diphosphate as the "universal" substrate of monoterpene synthases, in tomato glands neryl diphosphate serves as a precursor for the synthesis of monoterpenes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Old substrates for new enzymes of terpenoid biosynthesis.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10402-3. doi: 10.1073/pnas.0905226106. Epub 2009 Jun 24. Proc Natl Acad Sci U S A. 2009. PMID: 19553206 Free PMC article. No abstract available.
References
-
- Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol. 2006;9:297–304. - PubMed
-
- Croteau R, Felton M, Ronald RC. Biosynthesis of monoterpenes: Preliminary characterization of i-endo-fenchol synthetase from fennel (Foeniculum vulgare) and evidence that no free intermediate is involved in the cyclization of geranyl pyrophosphate to the rearranged product. Arch Biochem Biophys. 1980;200:534. - PubMed
-
- Croteau R, Karp F. Biosynthesis of monoterpenes: Preliminary characterization of bornyl pyrophosphate synthetase from sage (Salvia officinalis) and demonstration that geranyl pyrophosphate is the preferred substrate for cyclization. Arch Biochem Biophys. 1979;198:512–522. - PubMed
-
- Croteau R, Felton M. Conversion of [1-3H2,G-14C]geranyl pyrophosphate to cyclic monoterpenes without loss of tritium. Arch Biochem Biophys. 1981;207:460–464. - PubMed
-
- Colby SM, Alonso WR, Katahira EJ, McGarvey DJ, Croteau R. 4S-limonene synthase from the oil glands of spearmint (Mentha spicata). cDNA isolation, characterization, and bacterial expression of the catalytically active monoterpene cyclase. J Biol Chem. 1993;268:23016–23024. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

