Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer
- PMID: 19487666
- PMCID: PMC2701033
- DOI: 10.1073/pnas.0903815106
Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer
Abstract
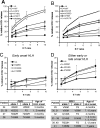
The pore-forming protein perforin is critical for defense against many human pathogens and for preventing a catastrophic collapse of immune homeostasis, manifested in infancy as Type 2 familial hemophagocytic lymphohistiocytosis (FHL). However, no evidence has yet linked defective perforin cytotoxicity with cancer susceptibility in humans. Here, we examined perforin function in every patient reported in the literature who lived to at least 10 years of age without developing FHL despite inheriting mutations in both of their perforin (PRF1) alleles. Our analysis showed that almost 50% of these patients developed at least 1 hematological malignancy in childhood or adolescence. The broad range of pathologies argued strongly against a common environmental or viral cause for the extraordinary cancer incidence. Functionally, what distinguished these patients was their inheritance of PRF1 alleles encoding temperature-sensitive missense mutations. By contrast, truly null missense mutations with no rescue at the permissive temperature were associated with the more common severe presentation with FHL in early infancy. Our study provides the first mechanistic evidence for a link between defective perforin-mediated cytotoxicity and cancer susceptibility in humans and establishes the paradigm that temperature sensitivity of perforin function is a predictor of FHL severity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. - PubMed
-
- Stepp SE, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. - PubMed
-
- Henter JI, Elinder G, Soder O, Ost A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991;80:428–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

