Sorting in early endosomes reveals connections to docking- and fusion-associated factors
- PMID: 19487677
- PMCID: PMC2691687
- DOI: 10.1073/pnas.0901444106
Sorting in early endosomes reveals connections to docking- and fusion-associated factors
Abstract
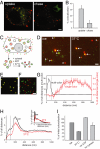
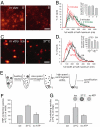
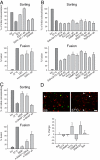
The early endosomes constitute a major sorting platform in eukaryotic cells. They receive material through fusion with endocytotic vesicles or with trafficking vesicles from the Golgi complex and later sort it into budding vesicles. While endosomal fusion is well understood, sorting is less characterized; the 2 processes are generally thought to be effected by different, unrelated machineries. We developed here a cell-free assay for sorting/budding from early endosomes, by taking advantage of their ability to segregate different cargoes (such as transferrin, cholera toxin subunit B, and low-density lipoprotein, LDL) into different carrier vesicles. Cargo separation required both carrier vesicle formation and active maturation of the endosomes. Sorting and budding were insensitive to reagents perturbing clathrin coats, coatomer protein complex-I (COPI) coats, dynamin, and actin, but were inhibited by anti-retromer subunit antibodies. In addition, the process required Rab-GTPases, phosphatidylinositol-3-phosphate, and, surprisingly, the docking factor early endosomal autoantigen 1 (EEA1). Sorting also required the function of the N-ethylmaleimide-sensitive factor (NSF), a well-known fusion cofactor, while it did not depend on preceding fusion of endosomes. We conclude that fusion, docking, and sorting/budding are interconnected at the molecular level.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
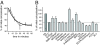
References
-
- Mills IG, Jones AT, Clague MJ. Regulation of endosome fusion. Mol Membr Biol. 1999;16:73–79. - PubMed
-
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. - PubMed
-
- Gruenberg J, Howell KE. Membrane traffic in endocytosis: Insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

