House dust mite allergen Der f 2-induced phospholipase D1 activation is critical for the production of interleukin-13 through activating transcription factor-2 activation in human bronchial epithelial cells
- PMID: 19487697
- PMCID: PMC2740436
- DOI: 10.1074/jbc.M109.010017
House dust mite allergen Der f 2-induced phospholipase D1 activation is critical for the production of interleukin-13 through activating transcription factor-2 activation in human bronchial epithelial cells
Abstract
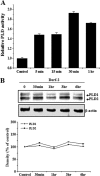
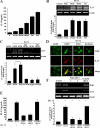
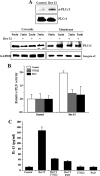
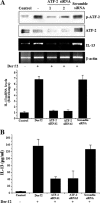
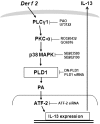
The purpose of this study was to identify the role of phospholipase D1 (PLD1) in Der f 2-induced interleukin (IL)-13 production. The major house dust mite allergen, Der f 2, increased PLD activity in human bronchial epithelial cells (BEAS-2B), and dominant negative PLD1 or PLD1 siRNA decreased Der f 2-induced IL-13 expression and production. Treatment of Der f 2 activated the phospholipase Cgamma (PLCgamma)/protein kinase Calpha (PKCalpha)/p38 MAPK pathway. Der f 2-induced PLD activation was attenuated by PLCgamma inhibitors (U73122 and PAO), PKCalpha inhibitors (RO320432 and GO6976), and p38 MAPK inhibitors (SB203580 and SB202190). These results indicate that PLCgamma, PKCalpha, and p38 MAPK act as upstream activators of PLD in Der f 2-treated BEAS-2B cells. Furthermore, expression and production of IL-13 increased by Der f 2 were also blocked by inhibition of PLCgamma, PKCalpha, or p38 MAPK, indicating that IL-13 expression and production are related to a PLCgamma/PKCalpha/p38 MAPK pathway. We found that activating transcription factor-2 (ATF-2) was activated by Der f 2 in BEAS-2B cells and activation of ATF-2 was controlled by PLD1. When ATF-2 activity was blocked with ATF-2 siRNA, Der f 2-induced IL-13 expression and production were decreased. Thus, ATF-2 might be one of the transcriptional factors for the expression of IL-13 in Der f 2-treated BEAS-2B cells. Taken together, PLD1 acts as an important regulator in Der f 2-induced expression and production of IL-13 through activation of ATF-2 in BEAS-2B cells.
Figures



References
-
- Oh J. W., Kim E. Y., Koo B. S., Lee H. B., Lee K. S., Kim Y. S., Han J. S. (2004) Exp. Mol. Med. 36, 486–492 - PubMed
-
- Thomas A. E., Platts-Mills T. A., Alanie L. De W. (1989) J. Allergy Clin. Immunol. 83, 416–427 - PubMed
-
- Chua K. Y., Doyle C. R., Simpson R. J., Turner K. J., Stewart G. A., Thomas W. R. (1990) Int. Arch Allergy Appl. Immunol. 91, 118–123 - PubMed
-
- Yuuki T., Okumura Y., Ando T., Yamakawa H., Suko M., Haida M., Okudaira H. (1991) Agric. Biol. Chem. 55, 1233–1238 - PubMed
-
- Holck A., Dale S., Sletten K. (1986) Allergy 41, 408–417 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

