Ecological genomics of marine picocyanobacteria
- PMID: 19487728
- PMCID: PMC2698417
- DOI: 10.1128/MMBR.00035-08
Ecological genomics of marine picocyanobacteria
Abstract
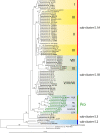
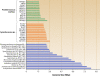
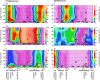
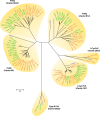
Marine picocyanobacteria of the genera Prochlorococcus and Synechococcus numerically dominate the picophytoplankton of the world ocean, making a key contribution to global primary production. Prochlorococcus was isolated around 20 years ago and is probably the most abundant photosynthetic organism on Earth. The genus comprises specific ecotypes which are phylogenetically distinct and differ markedly in their photophysiology, allowing growth over a broad range of light and nutrient conditions within the 45 degrees N to 40 degrees S latitudinal belt that they occupy. Synechococcus and Prochlorococcus are closely related, together forming a discrete picophytoplankton clade, but are distinguishable by their possession of dissimilar light-harvesting apparatuses and differences in cell size and elemental composition. Synechococcus strains have a ubiquitous oceanic distribution compared to that of Prochlorococcus strains and are characterized by phylogenetically discrete lineages with a wide range of pigmentation. In this review, we put our current knowledge of marine picocyanobacterial genomics into an environmental context and present previously unpublished genomic information arising from extensive genomic comparisons in order to provide insights into the adaptations of these marine microbes to their environment and how they are reflected at the genomic level.
Figures





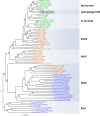
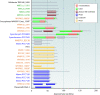

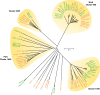

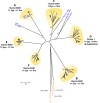


References
-
- Reference deleted.
-
- Ahlgren, N. A., G. Rocap, and S. W. Chisholm. 2006. Measurement of Prochlorococcus ecotypes using real-time polymerase chain reaction reveals different abundances of genotypes with similar light physiologies. Environ. Microbiol. 8441-454. - PubMed
-
- Aiba, H., and T. Mizuno. 1994. A novel gene whose expression is regulated by the response regulator, SphR, in response to phosphate limitation in Synechococcus species PCC7942. Mol. Microbiol. 1325-34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

