Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice
- PMID: 19487810
- PMCID: PMC2701856
- DOI: 10.1172/JCI35412
Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice
Abstract
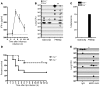
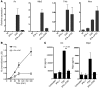
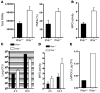
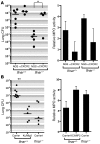
Influenza-related complications continue to be a major cause of mortality worldwide. Due to unclear mechanisms, a substantial number of influenza-related deaths result from bacterial superinfections, particularly secondary pneumococcal pneumonia. Here, we report what we believe to be a novel mechanism by which influenza-induced type I IFNs sensitize hosts to secondary bacterial infections. Influenza-infected mice deficient for type I IFN-alpha/beta receptor signaling (Ifnar-/- mice) had improved survival and clearance of secondary Streptococcus pneumoniae infection from the lungs and blood, as compared with similarly infected wild-type animals. The less effective response in wild-type mice seemed to be attributable to impaired production of neutrophil chemoattractants KC (also known as Cxcl1) and Mip2 (also known as Cxcl2) following secondary challenge with S. pneumoniae. This resulted in inadequate neutrophil responses during the early phase of host defense against secondary bacterial infection. Indeed, influenza-infected wild-type mice cleared secondary pneumococcal pneumonia after pulmonary administration of exogenous KC and Mip2, whereas neutralization of Cxcr2, the common receptor for KC and Mip2, reversed the protective phenotype observed in Ifnar-/- mice. These data may underscore the importance of the type I IFN inhibitory pathway on CXC chemokine production. Collectively, these findings highlight what we believe to be a novel mechanism by which the antiviral response to influenza sensitizes hosts to secondary bacterial pneumonia.
Figures


References
-
- Sethi S. Bacterial pneumonia. Managing a deadly complication of influenza in older adults with comorbid disease. Geriatrics. 2002;57:56–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

