Pathogenesis of holoprosencephaly
- PMID: 19487816
- PMCID: PMC2689134
- DOI: 10.1172/JCI38937
Pathogenesis of holoprosencephaly
Abstract
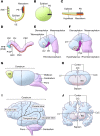
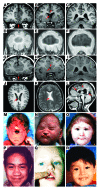
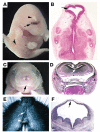
Holoprosencephaly (HPE), the most common human forebrain malformation, occurs in 1 in 250 fetuses and 1 in 16,000 live births. HPE is etiologically heterogeneous, and its pathology is variable. Several mouse models of HPE have been generated, and some of the molecular causes of different forms of HPE and the mechanisms underlying its variable pathology have been revealed by these models. Herein, we summarize the current knowledge on the genetic alterations that cause HPE and discuss some important questions about this disease that remain to be answered.
Figures


References
-
- Robb L., Tam P.P. Gastrula organiser and embryonic patterning in the mouse. Semin. Cell Dev. Biol. 2004;15:543–554. - PubMed
-
- Tam P.P., Loebel D.A. Gene function in mouse embryogenesis: get set for gastrulation. Nat. Rev. Genet. 2007;8:368–381. - PubMed
-
- Rallu M., Corbin J.G., Fishell G. Parsing the prosencephalon. Nat. Rev. Neurosci. 2002;3:943–951. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

